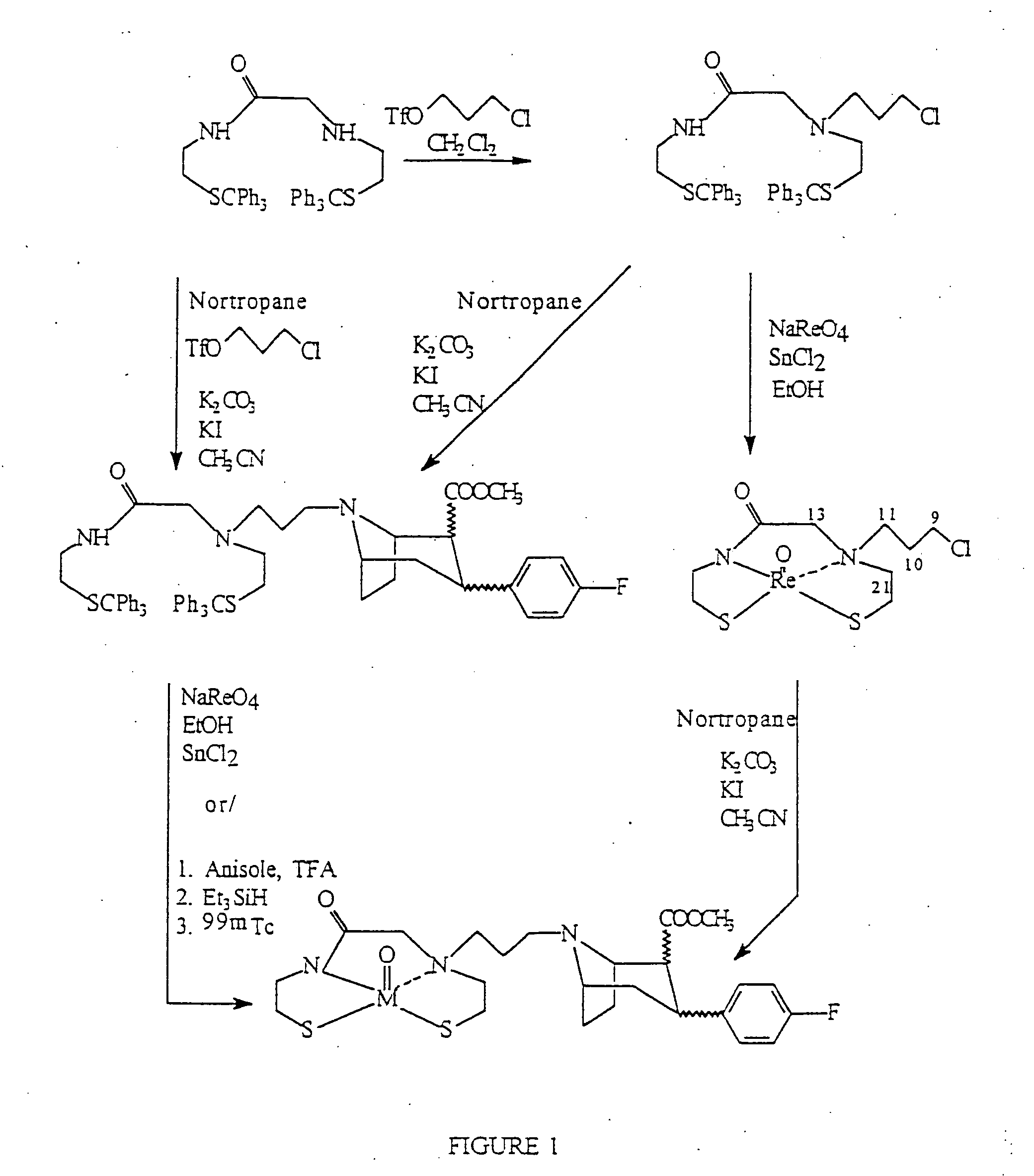
Boat tropanes
a technology of tropanes and boats, applied in the field of coordination complexes, can solve the problems of reducing striatal contrast, difficult to produce metal chelates which can cross the blood brain barrier, and difficult to quantify da
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
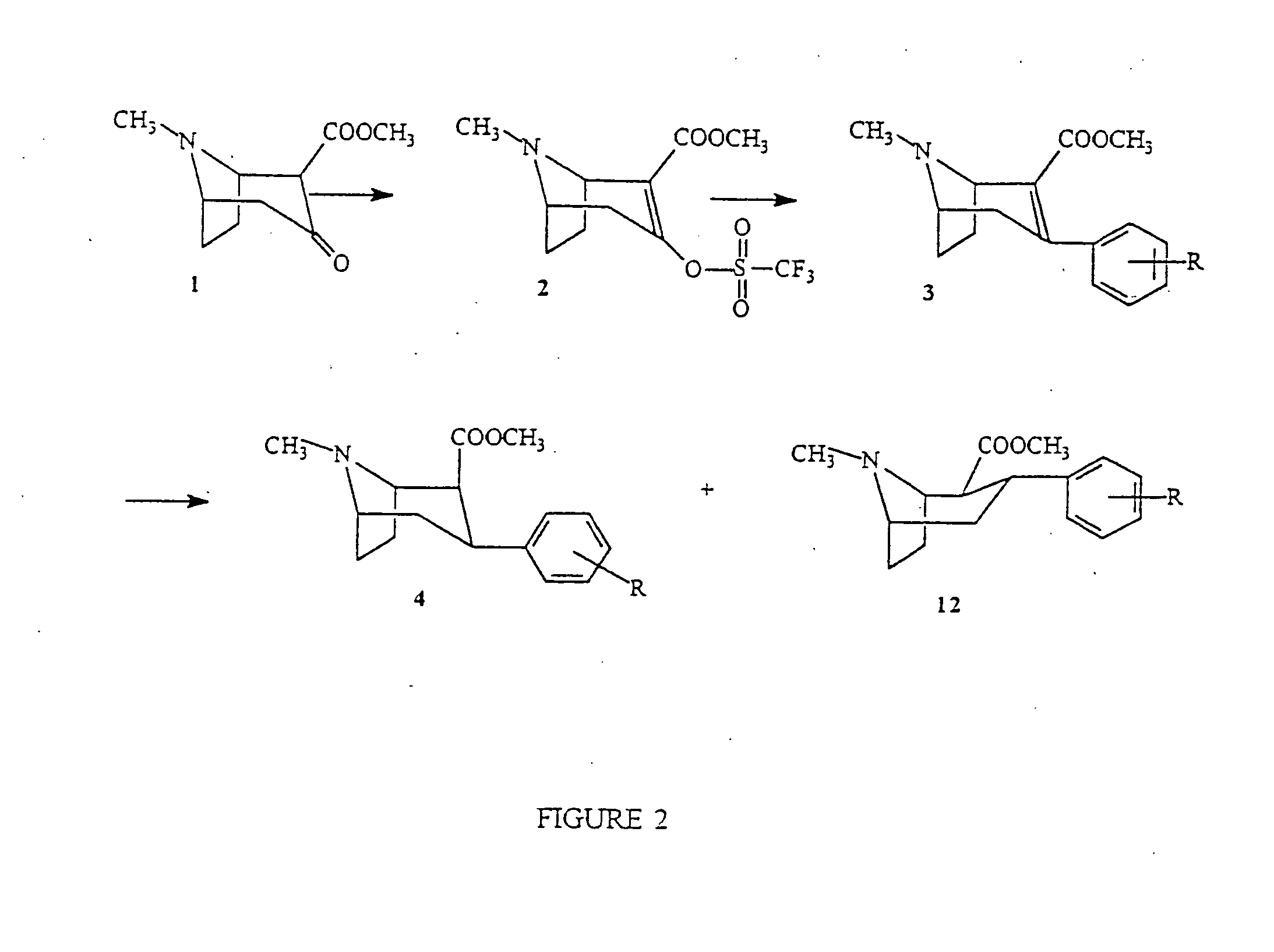
(1R)-2-(Methoxycarbonyl)-3-[[(trifluoromethyl)sulfonyl]oxy]trop-2-ene (Compound 2, FIG. 2)
[0087] (1R)-(−)-2-Methoxycarbonyl-3-tropinone, 1 {Meltzer et al., J. Med. Chem, 1994, 37, 2001} (1 g, 5.07 mmol) was dissolved in anhydrous THF (20 mL) and the resulting solution cooled to −78° C. A solution of sodium bistrimethylsilylamide (1 M, 5.58 mL, 5.58 mmol) was then added to the solution slowly. After 30 min, N-phenyltrifluoromethane sulfonamide (1.94 g, 5.43 mmol) was added. The resulting solution stirred for a further 45 min at −78° C. and then allowed to attain room temperature and stirred at room temperature for 2 h. All solvent was evaporated and the residue pumped to dryness. Column chromatography was performed on the residue (SiO2 60 g; 2%-16% methanol in ethyl acetate) and gave 1.62 g (97%) of a yellow oil which crystallized on standing.
[0088] Rf 0.65 (10% MeOH / EtOAc). 1H-NMR (CDCl3) δ 1.58 (m, 1H), 1.97 (m, 2H), 2.1-2.2 (m, 2H), 2.39 (s, 3H). 2.84 (dd, J=18, 4 Hz, 1H), 3.42 ...
example 2
(1R)-N-Methyl-2-methoxycarbonyl-3-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]oct-2-ene (Compound 3, FIG. 2)
[0089] (1R)-2-Methoxycarbonyl-3-[[(trifluoromethyl)sulfonyl]oxy] tropene 2 (620 mg, 1.88 mmol), LiCl (171 mg, 4.03 mmol), Pd2dba3 (69 mg, 0.075 mmol), aq. Na2CO3 (2.0 M, 2 mL), diethoxymethane (6.2 mL) were all charged to a flask and stirred vigorously. To this solution was added 3,4-dichlorophenyl boronic acid (474 mg, 2.49 mmol). The reaction was then brought to reflux for 2 h and filtered through celite. The cake was washed with ether and the organic solution was washed with concentrated NH4OH. The washed solvent was dried with K2CO3, filtered, and evaporated. The residue was charged to a column (SiO2, 60 g, eluted with 5-6% Et3N / EtOAc) and gave 512 mg (83%) of a yellow oil which solidified upon standing.
[0090] Rf 0.56 (10% Et3N / EtOAc). IR (KBr) 2941, 1724, 1460, 1418, 1333, 1250, 1212, 1124 cm−1. 1H-NMR (CDCl3) δ 1.61 (m, 1H), 1.9-2.05 (m, 2H), 2.1-2.3 (m, 2H), 2.43 (s, 3H),...
example 3
(1R)-N-Methyl-2β-methoxycarbonyl-3β-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]octane (Compound 4 (R=3,4-Cl2), FIG. 2), and
(1R)-N-Methyl-2β-methoxycarbonyl-3α-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]octane (Compound 12 (R=3,4-Cl2), FIG. 2)
[0091] To (1R)-N-Methyl-2-methoxycarbonyl-3-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]oct-2-ene, 3 (4 g, 12.3 mmol) in THF (43 mL) at −78° C. was added SmI2 solution (0.1 M in THF, 400 mL, 40 mmol) dropwise. After 30 min at −78° C., MeOH (140 mL) was added and the resulting solution stirred at −78° C. for a further 1 h. The reaction was then quenched with TFA (28 mL) and water (285 mL), the cold bath was removed and the solution allowed to attain room temperature. The reaction was then made basic with NH4OH and diluted with ether and filtered through celite. The filter cake was washed with ether and all the organic phases were combined and washed with a sodium thiosulfate solution and then a brine solution. After drying with Na2SO4 the solution was fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com