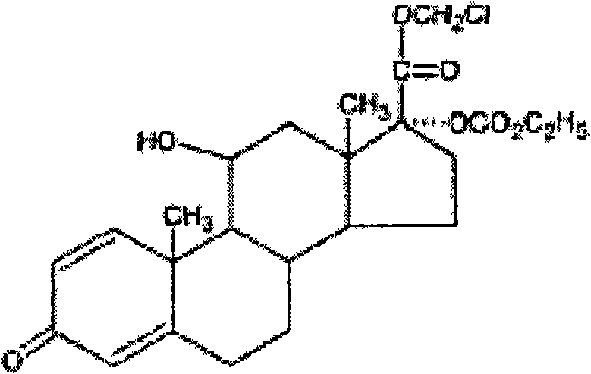
Medicament composition for eyes or nose, and uses thereof
A composition, ear and nose technology, applied in the directions of drug combination, drug delivery, antipyretic drugs, etc., can solve the problems of incomparability of chemical properties and physical properties, and the effect of levofloxacin and loteprednol etabonate cannot be inferred from this.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
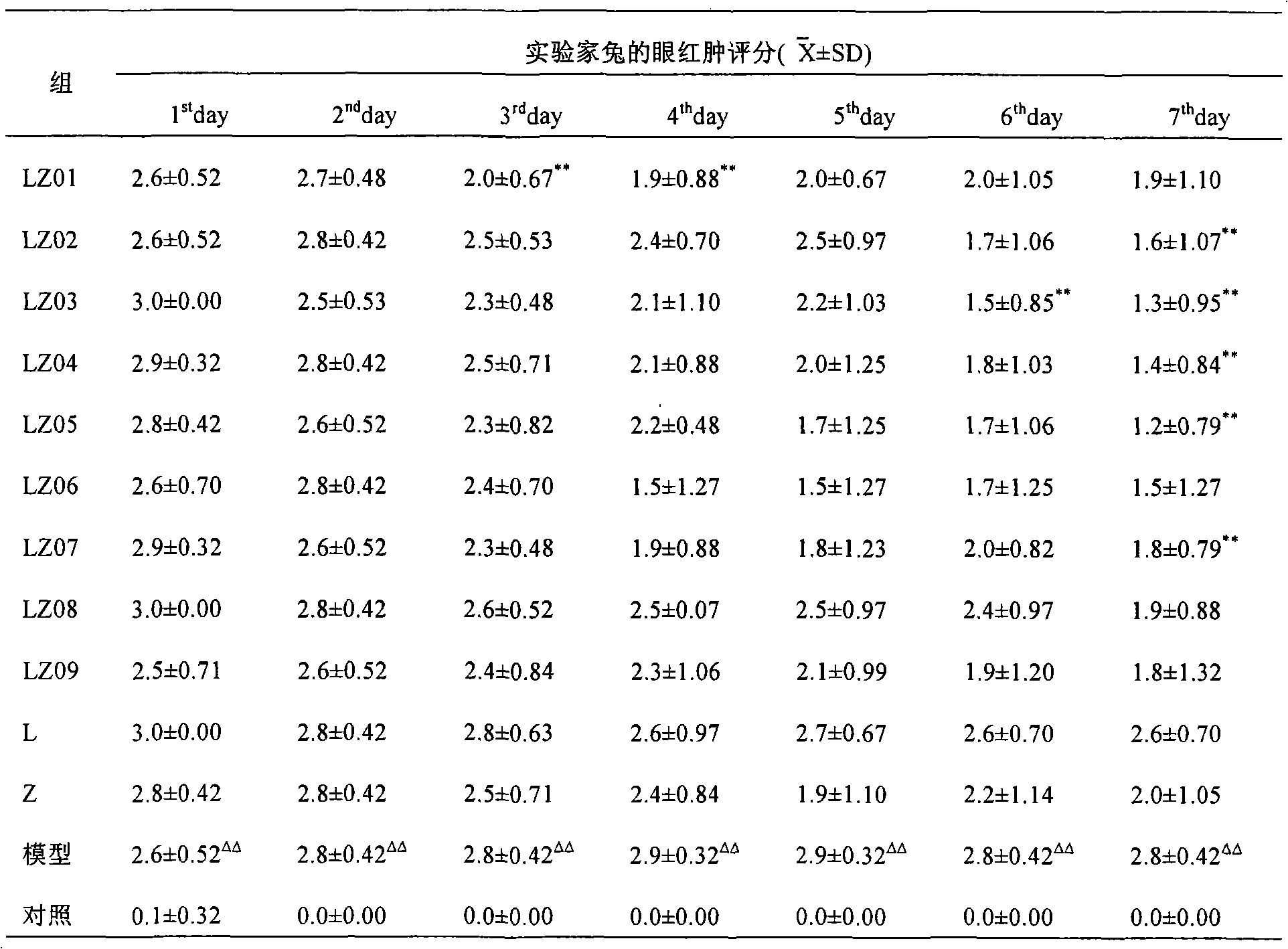
[0044] Example 1 Pharmacodynamic prescription screening test of levofloxacin loteprednol etabonate series concentration eye drops in rabbits.
[0045] 1. The purpose of the experiment: by instilling different proportions of levofloxacin loteprednol etabonate eye drops into the eyelids of rabbits after simulated incision surgery, to observe the antibacterial and anti-inflammatory effects of different concentration ratios of the liquids to prevent postoperative infection, Compare the difference in efficacy between the designed dose and other doses.
[0046] 2. Experimental materials
[0047] 1) Experimental samples
[0048] Levofloxacin loteprednol etabonate eye drops (LZ01-09), batch number: 060701.
[0049] LZ01: Specification: 5ml: 20mg loteprednol etabonate + 15mg levofloxacin;
[0050] LZ02: Specification: 5ml: 20mg loteprednol etabonate + 25mg levofloxacin;
[0051] LZ03: Specification: 5ml: 20mg loteprednol etabonate + 35mg levofloxacin;
[0052] LZ04: Specification:...
Embodiment 2
[0115] Embodiment 2, preparation
[0116] Table 7. Prescription (unit g / 200ml)
[0117]
[0118] Feed according to the ratio stated in the above prescription
[0119] 2. The preparation process of the pharmaceutical composition of the present invention to make suspension eye drops is as follows:
[0120] (1) sterilize the bottle for subsequent use;
[0121] (2) Loteprednol etabonate is microcrystallized and pulverized through a 200-mesh sieve, and sterilized by radiation for subsequent use;
[0122] (3) Dissolve each auxiliary material except loteprednol etabonate and levofloxacin in 80% water for injection, and dissolve levofloxacin in it after forming a clear solution to form a light yellow-green solution;
[0123] (4) Filter the above solution through a microporous membrane, and sterilize it by circulating steam at 100° C. for 30 minutes before use;
[0124] (5) Disperse the sterilized loteprednol etabonate in the standby liquid in a class 100 clean area, and dispers...
Embodiment 3
[0132] Embodiment 3, the influence of levofloxacin loteprednol etabonate compound (expressed in LZ) on rabbit intraocular pressure
[0133] 20 healthy rabbits were randomly divided into 4 groups (respectively LZ-H high-dose group, LZ-M medium-dose group, LZ-L low-dose group, negative control group), 5 in each group. Both eyes were instilled with the LZ05 proportioning eye drops described in Example 1, LZ-H, LZ-M, and LZ-L were administered 4, 2, and 1 drops respectively, and the negative control group (control) was instilled with 2 drops of vehicle . At 0 min, 30 min, 60 min, 120 min, 180 min, 240 min, 300 min, 360 min of administration, use YZ7A intraocular pressure meter to measure the intraocular pressure under tetracaine anesthesia. The above method reference can be found in the literature: Intraocular pressure, β-occlusion Action and dynamic changes of drug concentration in the intraocular fluid, Journal of ocular pharmacology, 1989, 5:271-279 . The data results are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com