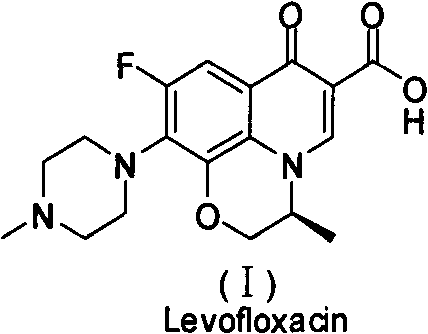
Method for preparing levofloxacin
A technology of levofloxacin and synthesis method, applied in organic chemistry and other directions, can solve the problems of poor atom economy, many three wastes, low yield and the like, and achieve the effects of reducing production cost, improving reaction yield and shortening reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be further described below in conjunction with specific embodiments.
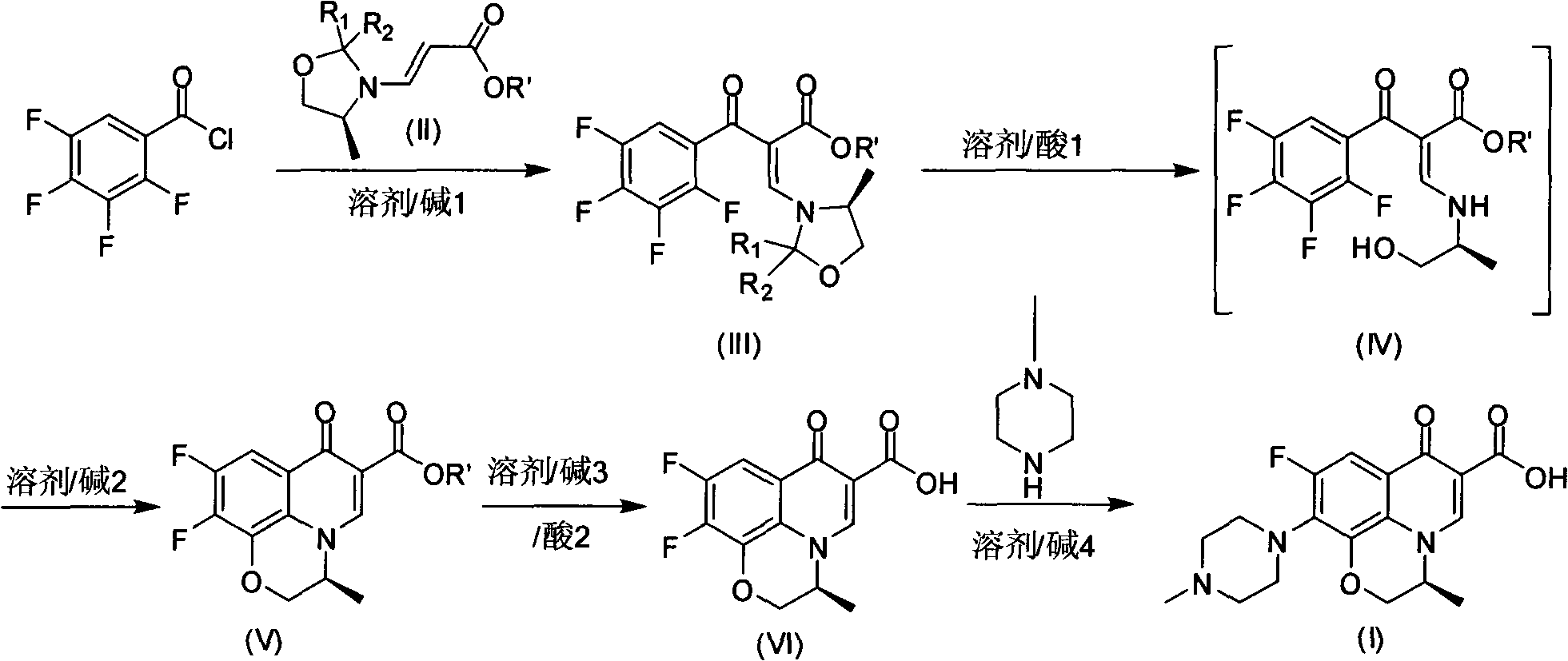
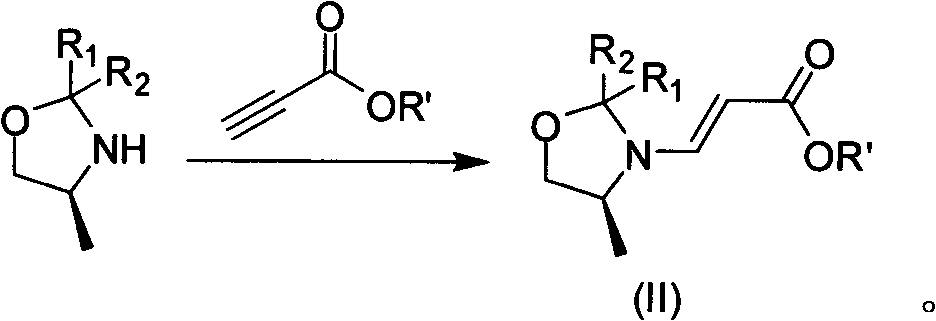
[0025] It uses tetrafluorobenzoyl chloride as raw material to prepare S-9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3- de]-[1,4]-benzoxazine-6-carboxylic acid (compound VI), then react with base 4 in an organic solvent to obtain levofloxacin, characterized in that S-9,10-difluoro -2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de]-[1,4]-benzoxazine-6-carboxylic acid is synthesized as follows :
[0026] a) Under stirring at room temperature, add base 1 and tetrafluorobenzoyl chloride to the solution of compound II, raise the temperature to 50-90°C, and stir for 2-5 hours to obtain a solution of compound III; add acid 1 under stirring In the solution of compound III, stir at 20-50°C for 10-60 minutes to obtain a solution of compound IV; add an appropriate amount of water to the solution of compound IV to separate layers, extract, and dry to obtain an organic layer of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com