Preparation method of cilastatin sodium impurity C
A technology for cilastatin sodium and impurities, which is applied in the field of preparation of cilastatin sodium impurity C, can solve problems such as affecting drug efficacy, and achieve the effects of low cost, optimized synthesis process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
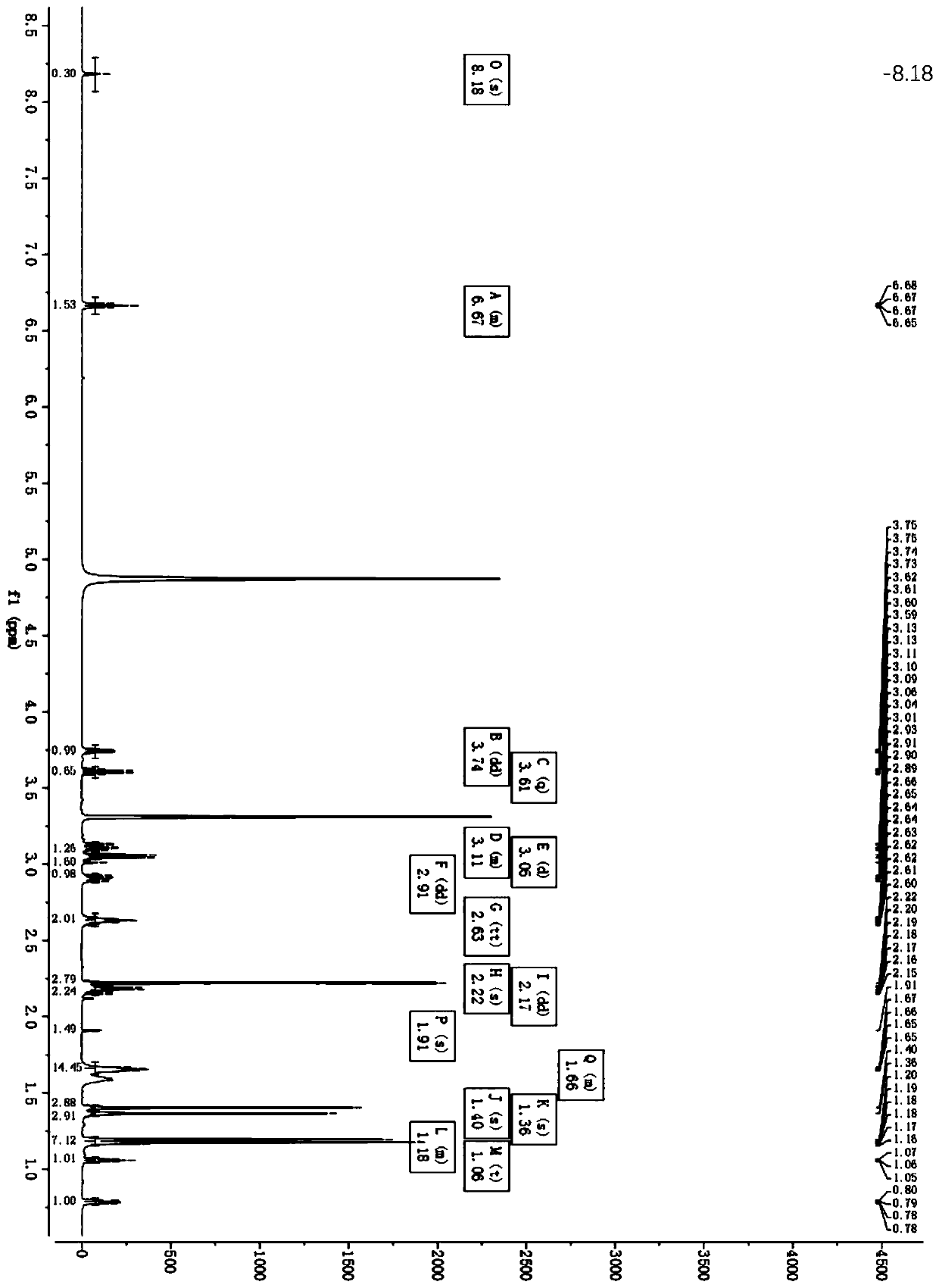
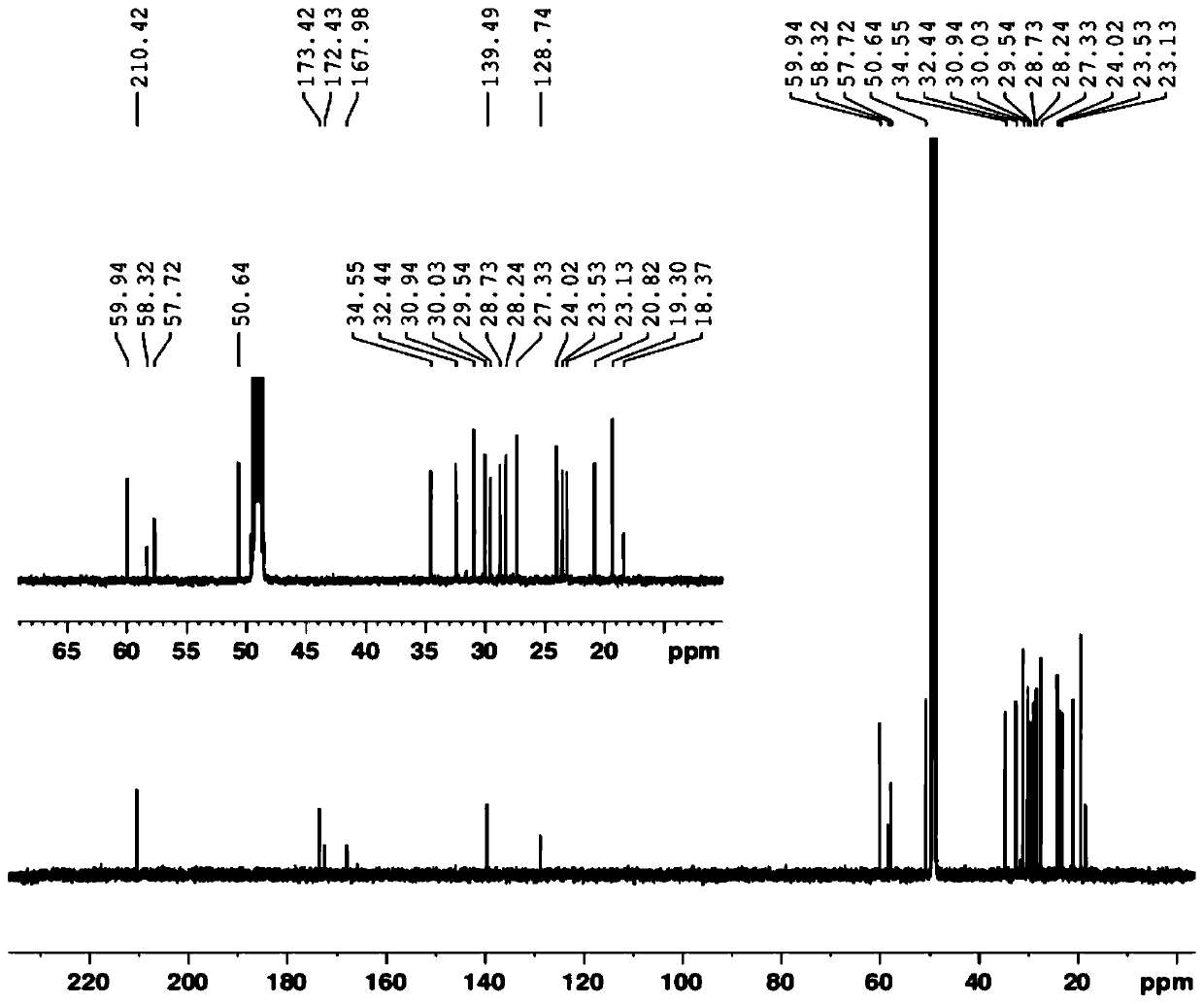
Image
Examples
Embodiment 1
[0055] (1) Methanol and DMSO are mixed into solvent according to the volume ratio of 1:1, and the former drug of dissolving cilastatin sodium makes the final concentration of cilastatin sodium be 250mg / ml, as need testing solution; Carry out high-pressure liquid chromatography mass spectrometry to obtain the peak entry time and peak exit time of cilastatin sodium impurity C;
[0056] High performance liquid chromatography-mass spectrometer: Thermo LCQ FLEET; Chromatographic column: Agilent ZORBAX SB-Aq, 4.6×250mm, 5μm;
[0057] Mobile phase A is an aqueous solution containing 0.1% formic acid; mobile phase B is acetonitrile;
[0058] Gradients are set to:
[0059]
[0060] The flow rate is 1mL / min; the detection wavelength is 210nm; the injection volume: 20μl.
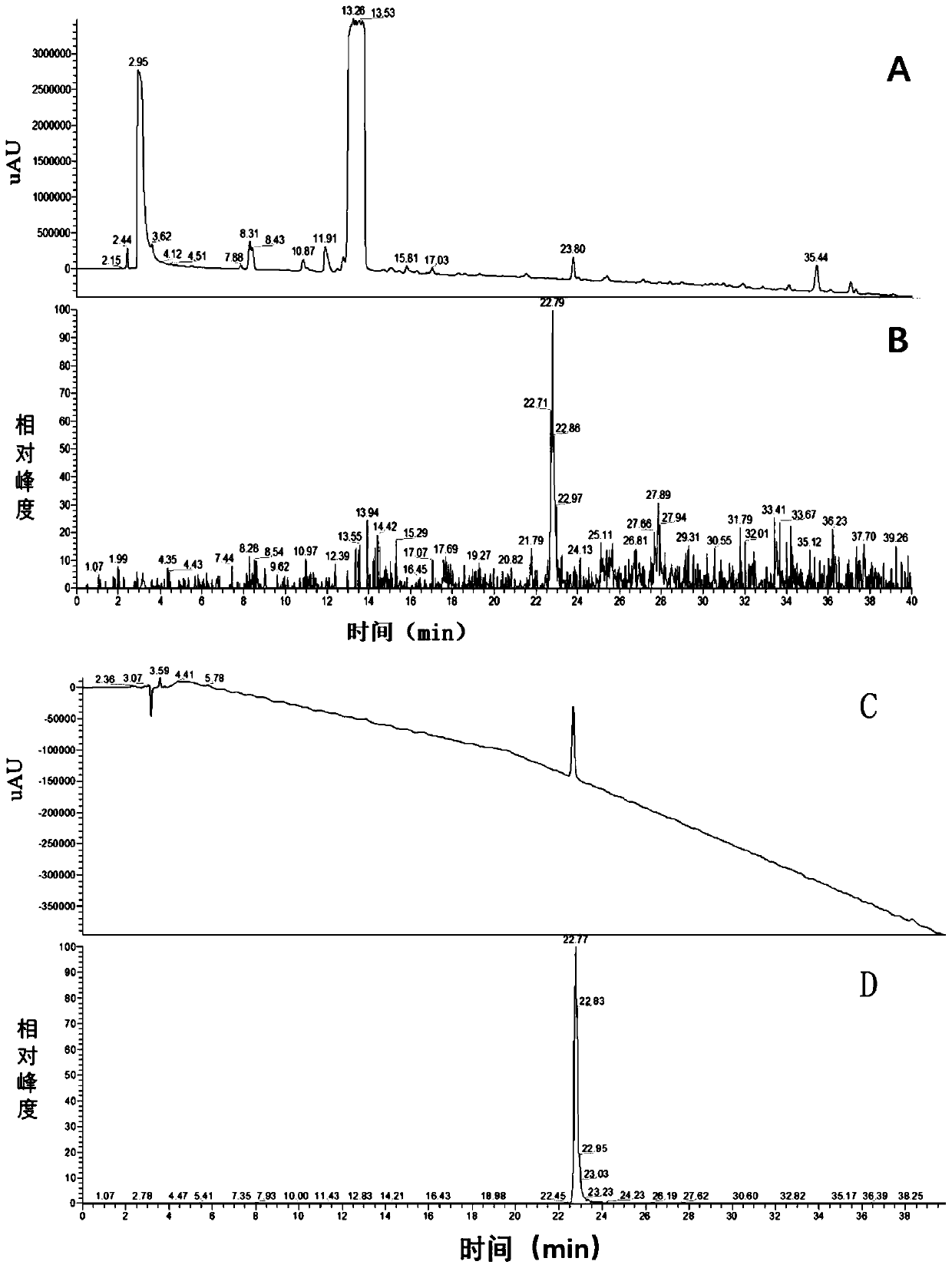
[0061] From the obtained chromatogram, the peak entry time and peak exit time of cilastatin sodium impurity C are 64.5, 65.4min respectively. The high-pressure liquid chromatography-mass spectrometry in this part...
Embodiment 2
[0079] (1) methanol and DMSO are mixed into a solvent according to the volume ratio of 1:1.2, and the former drug of cilastatin sodium is dissolved so that the final concentration of cilastatin sodium is 240mg / ml, as the test solution; Carry out high-pressure liquid chromatography mass spectrometry to obtain the peak entry time and peak exit time of cilastatin sodium impurity C;
[0080] High pressure liquid chromatography mass spectrometry parameters:
[0081] High performance liquid chromatography-mass spectrometer: Thermo LCQ FLEET; Chromatographic column: Agilent ZORBAX SB-Aq4.6×250mm, 5μm;
[0082] Mobile phase A is an aqueous solution containing 0.1% formic acid; mobile phase B is acetonitrile;
[0083] Gradients are set to:
[0084]
[0085] The flow rate is 1mL / min; the detection wavelength is 210nm; the injection volume: 20μl.
[0086] From the obtained chromatogram, the peak entry time and peak exit time of cilastatin sodium impurity C are 64.5, 65.4min respect...
Embodiment 3
[0101](1) methanol and DMSO are mixed into solvent according to the volume ratio of 1:1.5, and the former medicine of dissolving cilastatin sodium makes the final concentration of cilastatin sodium be 255mg / ml, as need testing solution; Carry out high-pressure liquid chromatography mass spectrometry to obtain the peak entry time and peak exit time of cilastatin sodium impurity C;
[0102] High pressure liquid chromatography mass spectrometry parameters:
[0103] High performance liquid chromatography-mass spectrometer: Thermo LCQ FLEET; Chromatographic column: Agilent ZORBAX SB-Aq, 4.6×250mm, 5μm;
[0104] Mobile phase A is an aqueous solution containing 0.1% formic acid; mobile phase B is acetonitrile;
[0105] Gradients are set to:
[0106]
[0107] The flow rate is 1mL / min; the detection wavelength is 210nm; the injection volume: 20μl.
[0108] From the obtained chromatogram, the peak entry time and peak exit time of cilastatin sodium impurity C are 64.5, 65.4min resp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com