Linked disulfide group substituted deuteroporphyrin, metal complexes, preparation method and uses thereof
A metal complex and hypoporphyrin technology is applied in the preparation and application of metallohypoporphyrin compounds, and can solve the problems of few studies on metallohypoporphyrin derivatives, poor catalyst selectivity, and low cyclohexane conversion rate. , to achieve the effect of short oxidation reaction time, less catalyst dosage, and improved selectivity of alcohols and ketones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
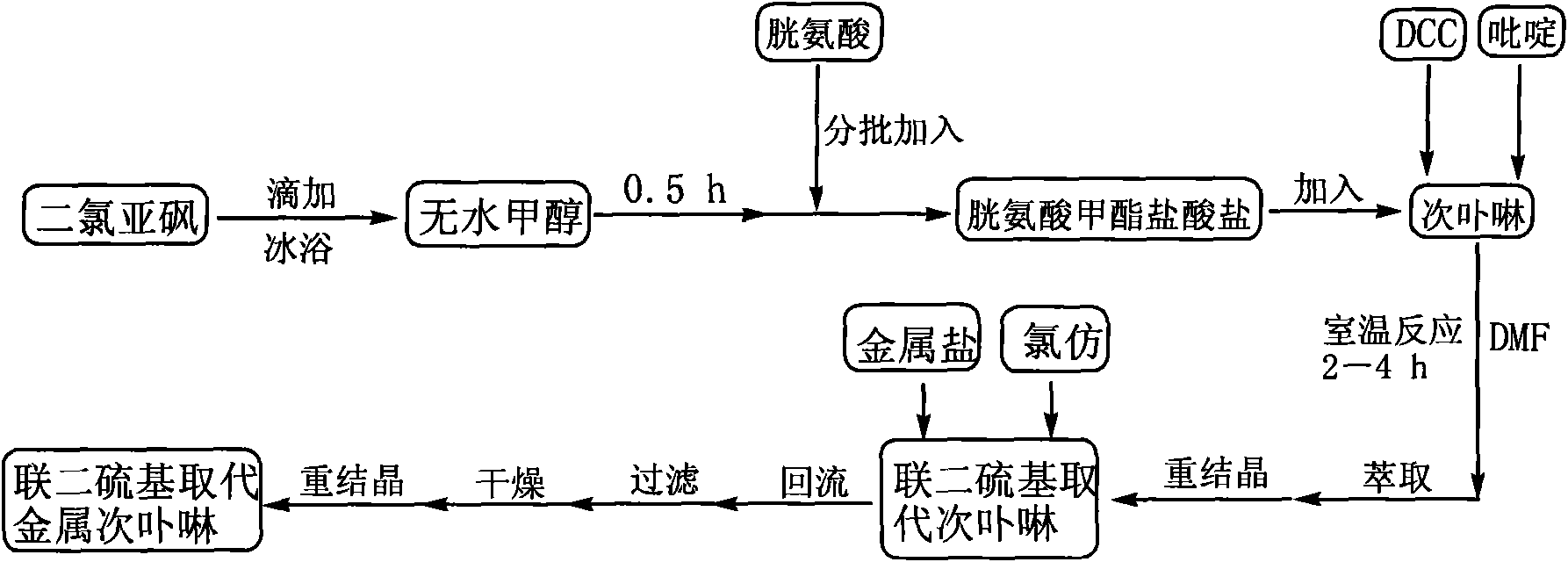
[0028] combine figure 1 , the preparation method of dithio-substituted hypoporphyrin of the present invention, comprises the following steps:
[0029] In the first step, slowly add thionyl chloride dropwise to a reactor containing anhydrous methanol under ice-bath conditions, and then add cystine powder in batches to the reactor. After the addition, react overnight at room temperature, distill off methanol and excess thionyl chloride to obtain a white cystine hydrochloride solid. Wherein, the molar ratio of thionyl chloride to cystine is 3:1-8:1.
[0030] In the second step, add hypoporphyrin, cystine methyl ester hydrochloride, dicyclohexylcarbodiimide (DCC), N, N-dimethylformamide (DMF) in the reactor, and then drop pyridine As a catalyst, stir the reaction at room temperature, the reaction time is 8 ~ 24h. After completion, 200 mL of saturated NaCl aqueous solution was added, and the aqueous layer was extracted with dichloromethane; after drying and recrystallization, a ...
Embodiment 1
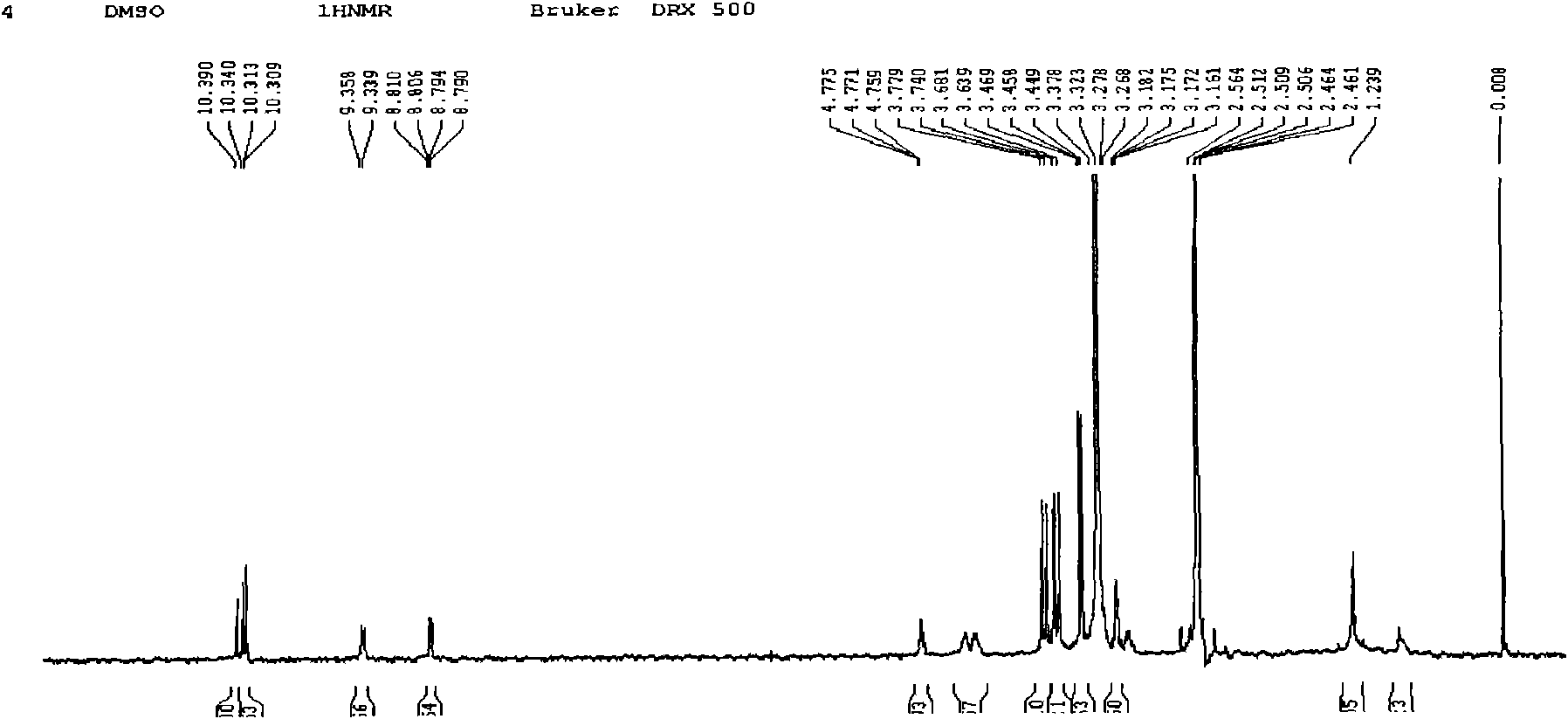
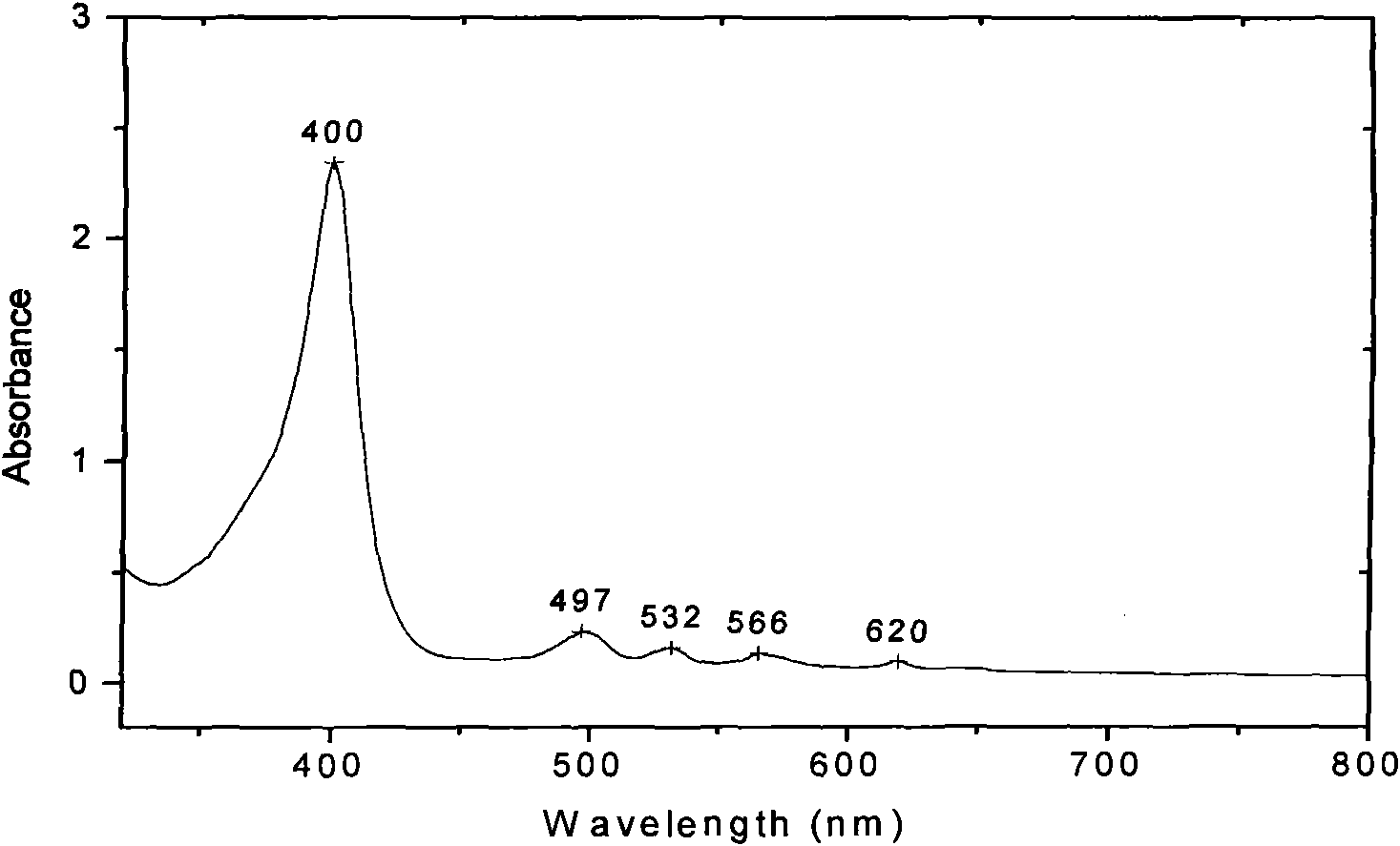
[0035]Example 1: In the first step, 5 mL of thionyl chloride was slowly added dropwise to a three-necked flask containing 100 mL of anhydrous methanol under ice-bath conditions, and then 2.0 g of cystine powder was added in batches to the three-necked flask. After the addition, react overnight at room temperature, distill methanol and excess thionyl chloride to obtain 2.48g of white cystine hydrochloride solid with a yield of 98%; in the second step, add 0.3g of cystine hydrochloride to a three-necked flask Hypoporphyrin, 0.18g cystine methyl ester hydrochloride, 0.34g dicyclohexylcarbodiimide (DCC), 80mL N, N-dimethylformamide (DMF), then dropwise added 1.5mL pyridine as a catalyst, The reaction was stirred at room temperature for 8.0 h. After completion, 200 mL of saturated NaCl aqueous solution was added, and the aqueous layer was extracted with dichloromethane. After drying and recrystallization, 0.16 g of reddish-brown powdery dithio-substituted hypoporphyrin solid was ob...
Embodiment 2
[0036] Embodiment 2: The other steps are the same as in Example 1. In the first step, under ice bath conditions, slowly add 5 mL of thionyl chloride dropwise to a three-necked flask with 100 mL of anhydrous methanol, and then add in batches to the three-necked flask Cystine powder 3.5g. After the addition, react overnight at room temperature, distill off methanol and excess thionyl chloride to obtain 4.3 g of white cystine hydrochloride solid with a yield of 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com