Preparation method of ribofuranose phosphate derivative
A technology of ribofuranose phosphate and derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of difficult control of purity, long process route, cumbersome operation, etc., to avoid excessive losses, Purity is easy to control, easy to control the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
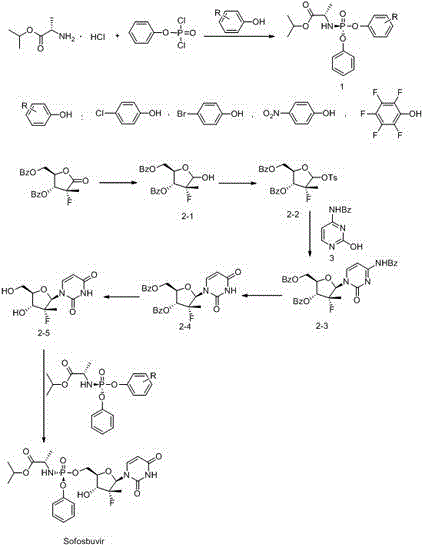
[0035] Preparation of intermediate formula 1:
[0036] Add 200mL of dichloromethane, 50g of L-alanine isopropyl hydrochloride and 38.3g of DIPEA into the reaction flask, stir, cool down to -78°C, react for 30min, then add dichloromethane of phenol dichlorophosphate dropwise solution (63g / 120mL); after dropping, stir the reaction for 30min, then raise the temperature to -10°C for 3 hours. Dissolve 55.4g of perfluorophenol and 35.2g of DIPEA in 200mL of dichloromethane, and add dropwise to the above reaction solution; after dropping, stir the reaction, and monitor the reaction by TLC; after the reaction is completed, filter with suction, and wash the filter cake with 50mL of dichloromethane , combine the organic phases, concentrate under reduced pressure, then dissolve and beat with 500mL methyl tert-butyl ether, filter with suction, wash the filter cake with 50mL methyl tert-butyl ether, combine the organic phases, and concentrate under reduced pressure to obtain 123.5g off...
Embodiment 2
[0050] Preparation of intermediate formula 1:
[0051] Add 200mL of dichloromethane, 50g of L-alanine isopropyl hydrochloride and 30g of triethylamine into the reaction flask, stir, cool down to -78°C, react for 30min, and then dropwise add the dichloride of phenol dichlorophosphate Methane solution (63g / 1200mL); after dropping, stir for 30 minutes, then raise the temperature to -10°C for 3 hours. Dissolve 42.7g of 4-nitrophenol and 30.6g of triethylamine in 200mL of dichloromethane, and add dropwise to the above-mentioned reaction solution; after dropping, stir the reaction, and monitor the reaction by TLC; Wash with methyl chloride, combine the organic phases, concentrate under reduced pressure, dissolve and beat with 500 mL of isopropyl ether, filter with suction, wash the filter cake with 50 mL of isopropyl ether, combine the organic phases, and concentrate under reduced pressure to obtain 116.2 g of off-white crude product. The crude product was refined and purified ...
Embodiment 3
[0066] Preparation of intermediate formula 1:
[0067] Add 200mL toluene, 50g L-alanine isopropyl hydrochloride and 26.4g piperidine into the reaction flask, stir, cool down to -78°C, react for 30min, then add dropwise the toluene solution of phenol dichlorophosphate (63g / 120mL); after dropping, stir the reaction for 30min, then raise the temperature to -10℃ for 3 hours. Dissolve 46.5 g of 4-bromophenol and 25 g of piperidine in 100 mL of toluene, and add dropwise to the above-mentioned reaction solution; after dropping, stir the reaction, and monitor the reaction by TLC; after the reaction is completed, filter the filter cake with 50 mL of toluene, and combine the organic phase, concentrated under reduced pressure, and then dissolved in 500 mL of methyl tert-butyl ether for beating, suction filtered, and the filter cake was washed with 50 mL of methyl tert-butyl ether, the organic phases were combined and concentrated under reduced pressure to obtain 119.6 g of off-white...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com