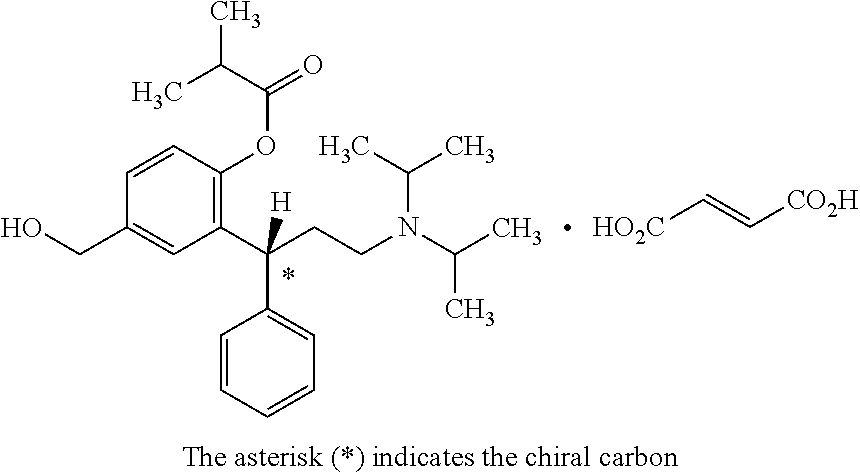
Stable compositions of fesoterodine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example-1
Pre-Lubrication of Excipients with Glyceryl Behenate
Fesoterodine Tablets Prepared According to the Composition Listed in Table 2 Using the Following Steps:
[0066]1. Pregelatinized starch, citric acid monohydrate, hydroxypropyl methylcellulose K100 CR, hydroxypropyl methylcellulose K100M CR, hydroxypropyl methylcellulose K4M, and colloidal silicon dioxide were sifted with a half quantity of glyceryl behenate through a mesh #40 sieve and dry blended for 15 minutes.[0067]2. 10% blend of step 1 and fesoterodine hydrogen fumarate were sifted together through a mesh #40 sieve.[0068]3. sifted materials of step 1 and step 2 were blended for 10 minutes.[0069]4. remaining half quantity of glyceryl behenate was sifted through a mesh #60 sieve.[0070]5. blend of step 3 was lubricated with glyceryl behenate of step 4.[0071]6. lubricated blend of step 5 was directly compressed into tablets.[0072]7. tablets of step 6 were film coated using an Opadry® dispersion.
TABLE 2Fesoterodine tablet composition...
example 3-5
[0077]
TABLE 4Fesoterodine tablet compositions prepared by granulation process:Example-3Example-4Example-5mg / Tabletmg / Tabletmg / Tablet(Dry (Wet(WetIngredientsgranulation)granulation)granulation)Intra-granular ingredientsFesoterodine fumarate8.008.008.00Pregelatinized starch147.44147.44147.44Hydroxypropyl72.0072.0072.00methylcelluloseK100 CRHydroxypropyl48.0048.0048.00methylcelluloseK100M CRHydroxypropyl24.0024.0024.00methylcelluloseK4MCitric acid0.660.660.66monohydrateColloidal9.909.909.90silicon dioxideGlyceryl behenate5.005.005.00GranulationPurified water—q.s.—Isopropyl alcohol——q.s.ExtragranularingredientsGlyceryl behenate5.005.005.00Core tablet weight320.0320.0320.0Film coatingOpadry ® blue10.0010.0010.00Film coated tablet weight330.00330.00330.00
Manufacturing Process for Example 3-5:
[0078]1. Pregelatinized starch, citric acid monohydrate, hydroxypropyl methylcellulose K100 CR, hydroxypropyl methylcellulose K100M CR, hydroxypropyl methylcellulose K4M and colloidal silicon dioxide ...
example 6
Fesoterodine Tablet Compositions Prepared by Direct Compression Process
[0082]
Ingredientmg / TabletFesoterodine fumarate8.00Pregelatinized starch116.10Hydroxypropyl72.00methylcellulose K 100 CRHydroxypropyl48.00methylcellulose K 100M CRHydroxypropyl24.00methylcellulose K 4M CRHydroxypropyl cellulose32.00Colloidal silicon dioxide9.90Glyceryl behenate10.00Core tablet weight320.00Film coatingOpadry ® blue10.00Coated tablet weight330.00
Manufacturing Process:
[0083]1. Pregelatinized starch, hydroxypropyl methylcellulose K 100 CR, hydroxypropyl methylcellulose K 100M CR, hydroxypropyl methylcellulose K4M CR, hydroxypropyl cellulose, colloidal silicon dioxide were sifted with a half quantity of glyceryl behenate through a mesh #40 sieve and blended for 15 minutes.
2. 10% of the blend of step 1 and fesoterodine fumarate were sifted together through a mesh #40 sieve.
3. sifted materials of step 1 and 2 were blended for 10 minutes.
4. remaining half quantity of glyceryl behenate was sifted through a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com