Heparin derivative-poloxamer temperature sensitive hydrogel and preparation method thereof
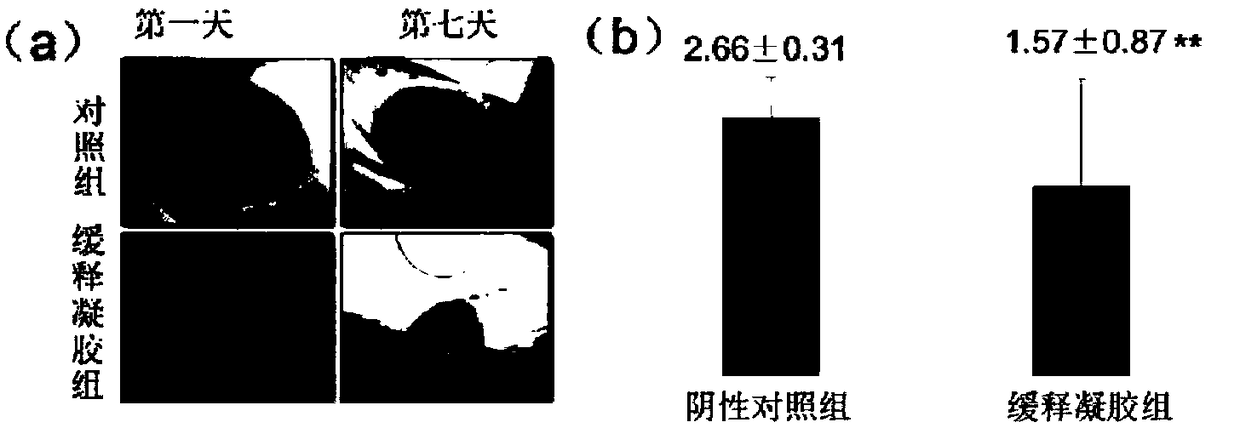
A technology of heparin derivatives and hydrogels, applied in the field of biomedical materials, can solve problems such as unapplied reports, reduce the number of dressing changes and improve compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
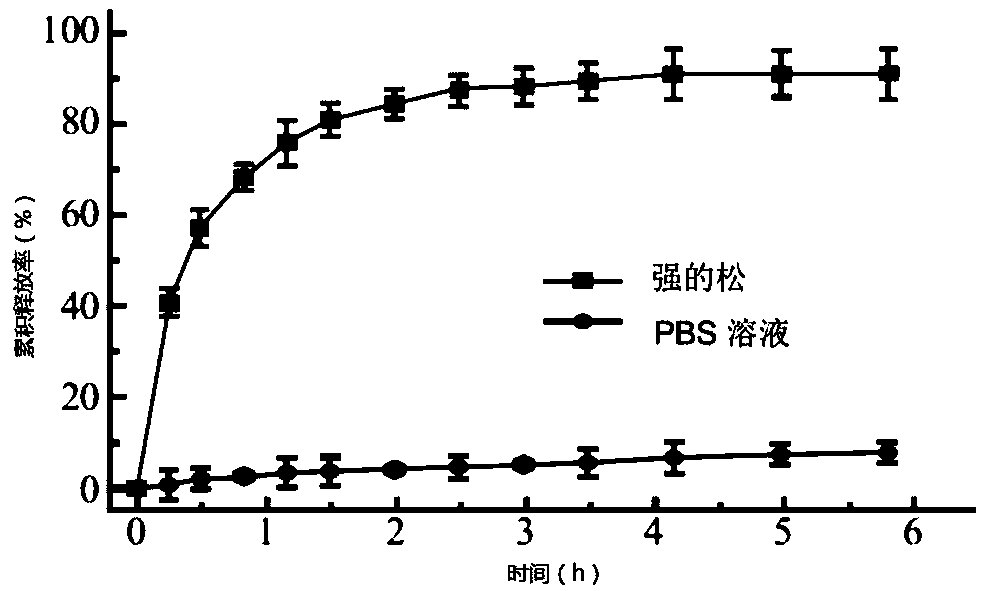
[0031] Preparation of heparin derivative-poloxamer thermosensitive hydrogel Weigh 15-90 mg of N-acetylated low molecular weight heparin and put it in a serum bottle, add 1 mL of PBS buffer solution (pH=7.0), prednisone drug PBS Buffer solution (mass concentration 5%); stir and dissolve at room temperature for 24 hours. Weigh poloxamer (100-300mg P407) and add the above low molecular weight heparin solution (V is 1-3mL), stir and dissolve at 4°C for 24h, put the vial of heparin-poloxamer mixture in a water bath, and heat slowly The temperature was raised, and the gelation temperature and gelation time were judged and determined by the inverted bottleneck method.
Embodiment 2
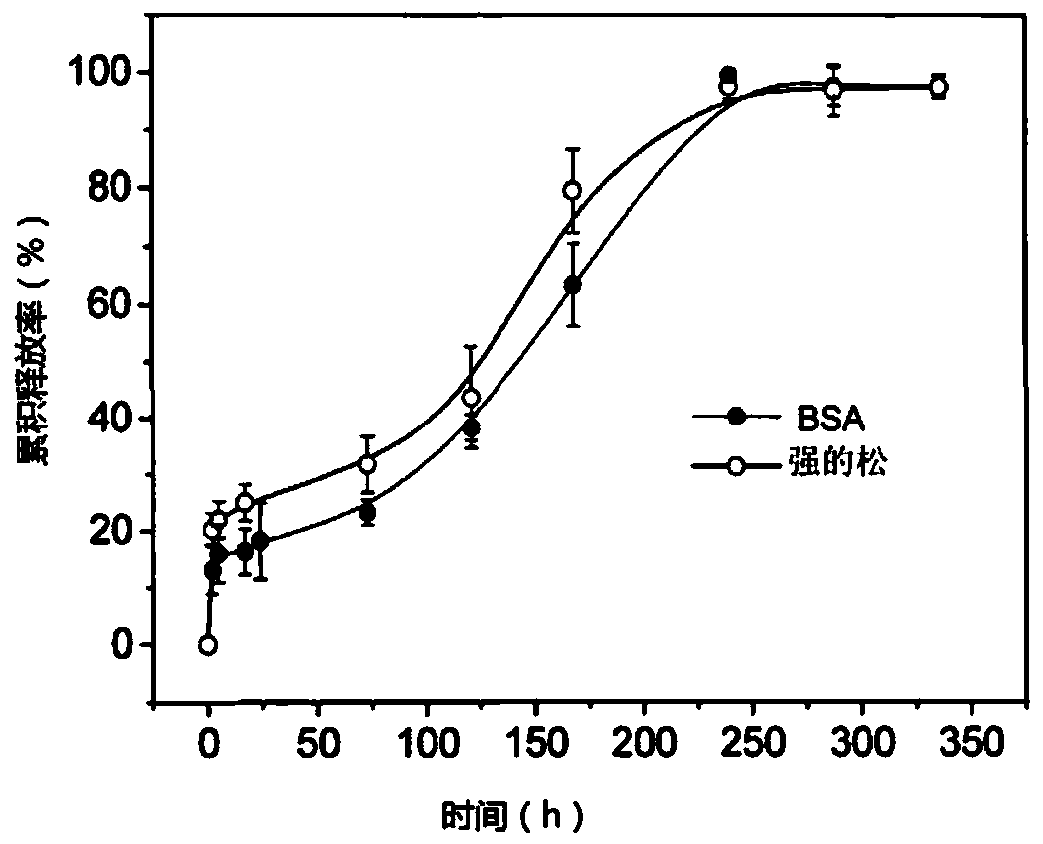
[0033] PLGA-heparin derivatives-poloxamer thermosensitive hydrogel was prepared with PLGA as the carrier material. Prednisone microspheres were prepared by the W / O / W double emulsion-drying method with simple process and convenient operation. The prednisone solution of 5% mass concentration is added in the dichloromethane solution of the PLGA of 10% mass concentration, forms colostrum after ultrasonic dispersion; With 6% PVA solution as aqueous dispersion medium, its inorganic salt mass concentration is 2% . Add 2mL of PVA solution dropwise to the above-mentioned emulsion, after homogenization, pour this solution into 8mL of PVA solution of the same concentration, and stir magnetically at room temperature for 3-5h to obtain the prednisone-PLGA colloidal solution.
[0034] Weigh 15-90 mg of N-acetylated low molecular weight heparin and put it in a serum bottle, add 1 mL of PBS buffer solution (pH=7.0), prednisone-PLGA drug colloid solution; stir and dissolve at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com