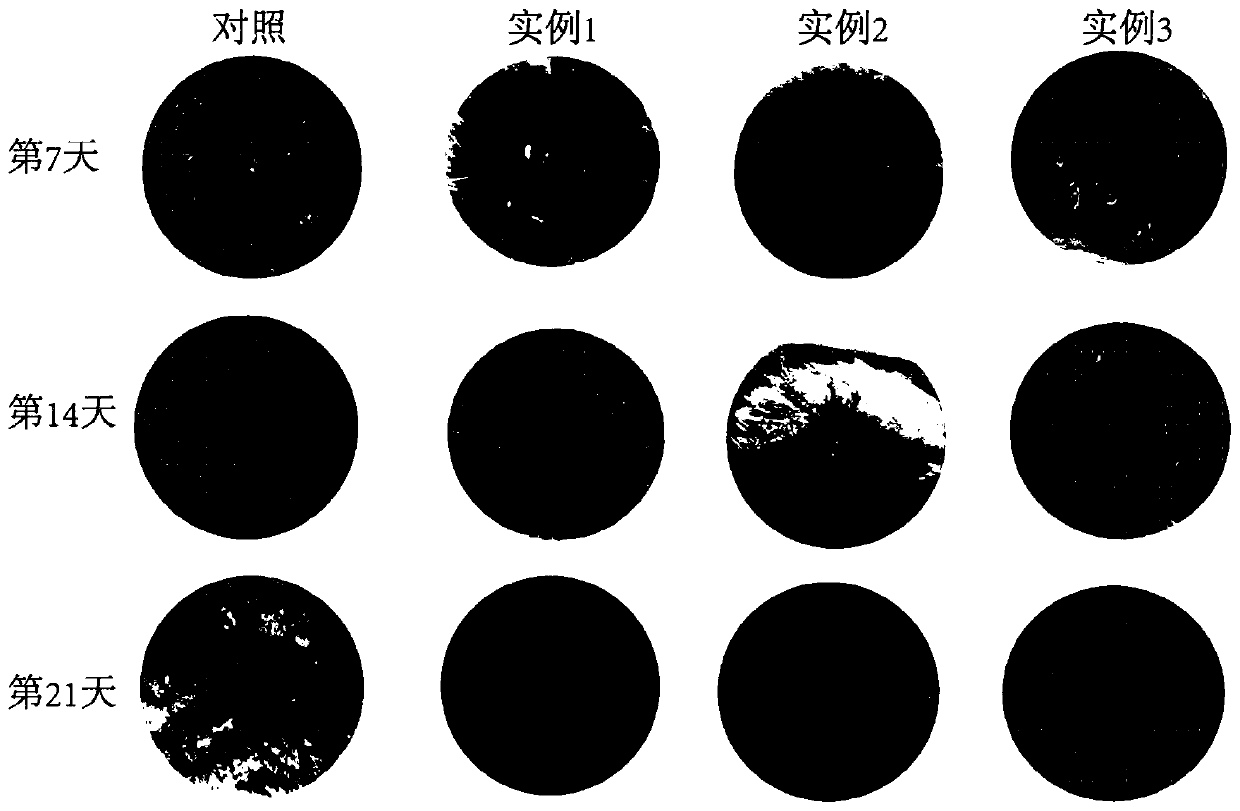
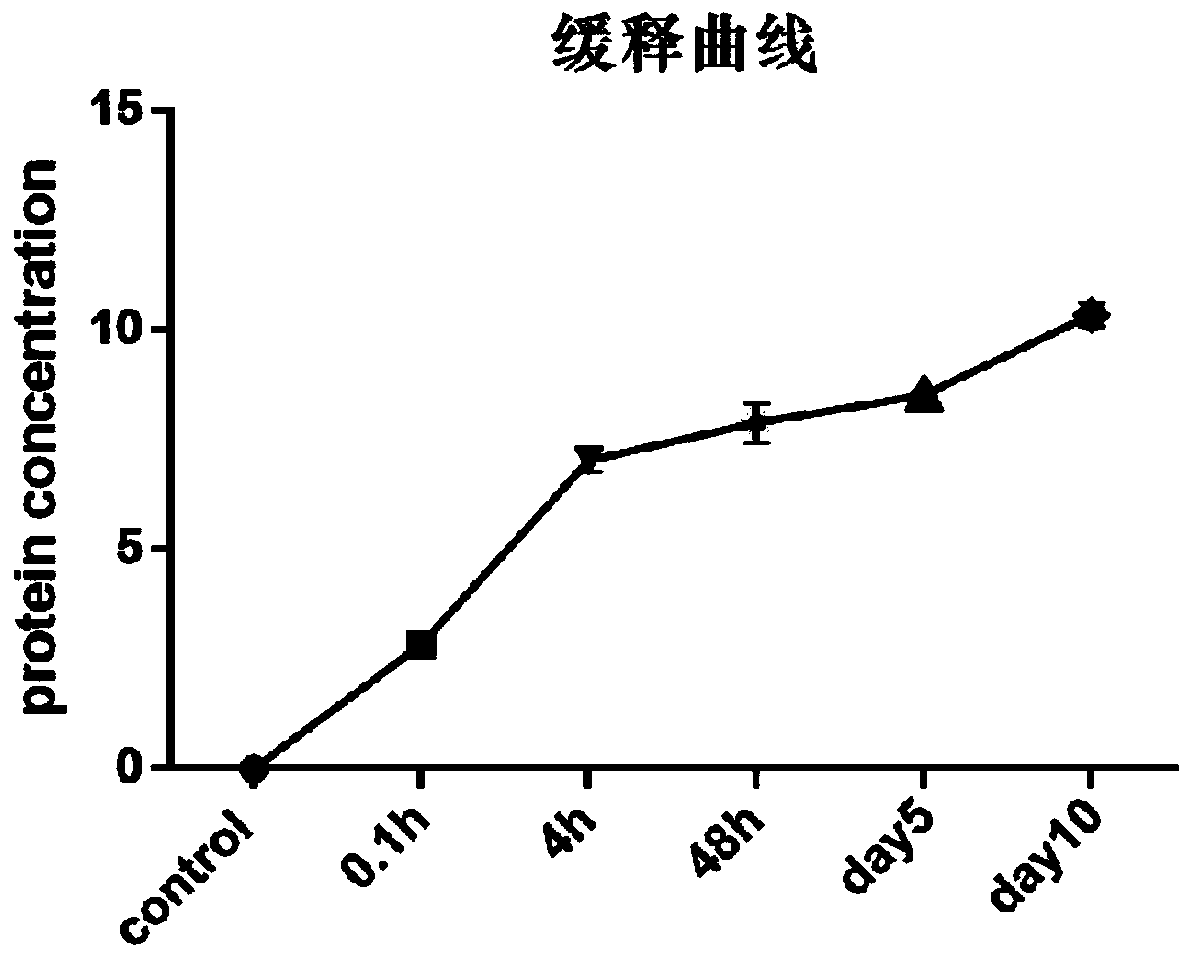
Preparation method of medical dressing
A stem cell and solution technology, used in medical science, absorbent pads, bandages, etc., can solve problems such as unsatisfactory effects, and achieve the effects of being conducive to wound repair, avoiding secondary trauma, and reducing the number of dressing changes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of PLGA sustained-release microspheres containing secretin
[0036] S1. Dissolve PLGA in dichloromethane to prepare a PLGA dichloromethane solution with a concentration of 10% (w / v);
[0037] S2. The stem cell secretin powder is dissolved in deionized water to prepare a stem cell secretin solution with a concentration of 2% (w / v). In this embodiment, the stem cell secretin is derived from human subtotipotent stem cells;
[0038] S3. Under the condition of vortexing and shaking, add the stem cell secretin solution dropwise to the PLGA dichloromethane solution and mix, and then phacoemulsify for 10 minutes under ice bath conditions to prepare W / O colostrum;
[0039] S4. Under the condition of vortex shaking, drop the W / O colostrum into the PVA aqueous solution with a concentration of 2.5% (w / v), and then intermittently use ultrasonic treatment under ice bath conditions to prepare W / O / W double breast; ultrasound conditions: 10-38% amplitude (418J), 10s pause every...
Embodiment 2
[0050] (1) Preparation of PLGA sustained-release microspheres containing secretin
[0051] S1. Dissolve PLGA in dichloromethane to prepare a PLGA dichloromethane solution with a concentration of 10% (w / v);
[0052] S2. The stem cell secretin powder is dissolved in deionized water to prepare a stem cell secretin solution with a concentration of 2.5% (w / v). In this embodiment, the stem cell secretin is derived from human subtotipotent stem cells;
[0053] S3. Under the condition of vortexing and shaking, add the stem cell secretin solution dropwise to the PLGA dichloromethane solution and mix, and then phacoemulsify for 10 minutes under ice bath conditions to prepare W / O colostrum;
[0054] S4. Under the condition of vortex shaking, drop the W / O colostrum into the PVA aqueous solution with a concentration of 3% (w / v), and then intermittently use ultrasonic treatment under ice bath conditions to make W / O / W double breast; ultrasound conditions: 10-38% amplitude (418J), 10s pause every 4s...
Embodiment 3
[0065] (1) Preparation of PLGA sustained-release microspheres containing secretin
[0066] S1. Dissolve PLGA in dichloromethane to prepare a PLGA dichloromethane solution with a concentration of 10% (w / v);
[0067] S2. The stem cell secretin powder is dissolved in deionized water to prepare a stem cell secretin solution with a concentration of 3% (w / v). In this embodiment, the stem cell secretin is derived from human subtotipotent stem cells;
[0068] S3. Under the condition of vortexing and shaking, add the stem cell secretin solution dropwise to the PLGA dichloromethane solution and mix, and then phacoemulsify for 10 minutes under ice bath conditions to prepare W / O colostrum;
[0069] S4. Under the condition of vortex shaking, drop the W / O colostrum into the PVA aqueous solution with a concentration of 3.5% (w / v), and then intermittently use ultrasonic treatment under ice bath conditions to make W / O / W double breast; ultrasound conditions: 10-38% amplitude (418J), 10s pause every 4s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com