Methylprednisolone intermediate debrominated product and preparation method thereof
A technology for methylprednisolone and intermediates, applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve the problems of low product purity, long synthetic route, cumbersome operation, etc., and achieve high yield, The effect of short synthetic route and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
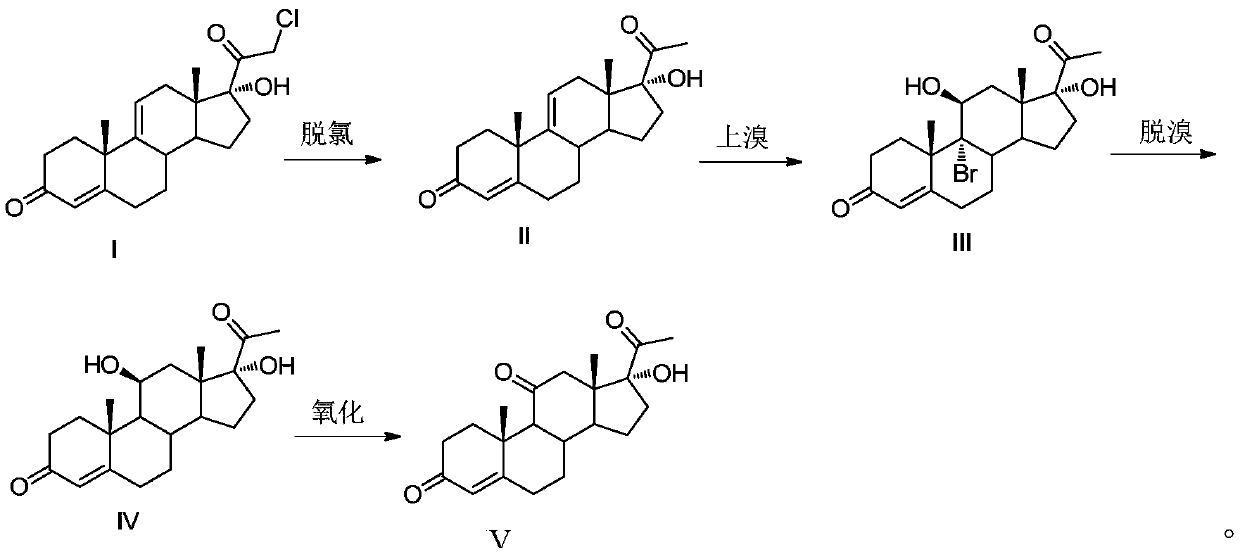
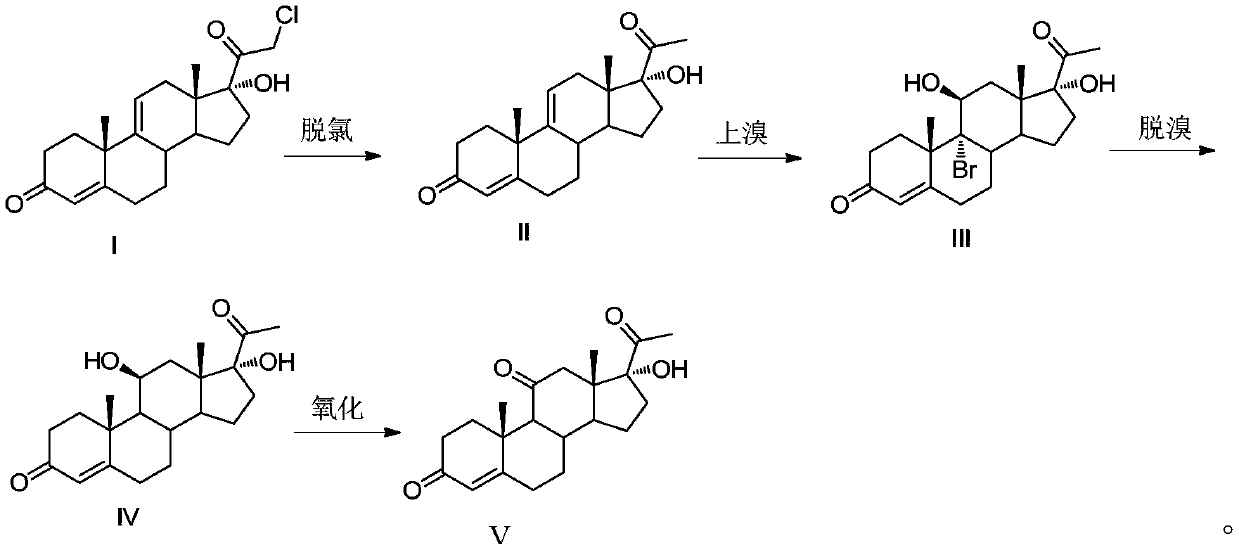
[0035] This embodiment provides a methylprednisolone intermediate and a preparation method thereof. The preparation method comprises the steps of:
[0036] (1) Dechlorination reaction: add 350ml of acetone, 50g of the compound shown in formula I, 15g of zinc powder, and 75ml (78.7g) of glacial acetic acid into the three-necked flask, and heat up to 50-55°C for reflux reaction for 6-7h; TLC Track the reaction. After the reaction is finished, cool the system down to below 35°C, add 350ml of dichloromethane, filter, and wash the filter cake with a small amount of dichloromethane; steam the filtrate to a paste, add 250ml of water, stir at room temperature for 1h, pump Filter, wash with water until neutral, and dry to obtain 42g of the compound shown in formula II. The yield of the compound shown in formula II is 92.8%, and the purity is 95.7% (HPLC). HR-ESI-Ms(M+H): 329.2;
[0037] (2) Bromine reaction: Add 400ml of acetone and 20ml of water into a 1L three-necked flask, cool to...
Embodiment 2
[0041] This embodiment provides a methylprednisolone intermediate and a preparation method thereof. The preparation method comprises the steps of:
[0042] (1) Dechlorination reaction: add 350ml of butanone, 50g of the compound shown in formula I, 20g of zinc powder, and 75ml (78.7g) of glacial acetic acid into the three-necked flask, and heat up to 50-55°C for 6-7h; TLC Track the reaction. After the reaction is finished, cool the system down to below 35°C, add 350ml of dichloromethane, filter, and wash the filter cake with a small amount of dichloromethane; steam the filtrate to a paste, add 250ml of water, stir at room temperature for 1h, pump Filter, wash with water to neutrality, dry to obtain the compound shown in 43.5g formula II, the yield of the compound shown in formula II is 96.1%, and purity is 95.2% (HPLC);
[0043] (2) Bromination reaction: Add 400ml of acetone and 20ml of water into a 1L three-necked flask, cool to -5 ~ 0°C, add 20g of fluoboric acid, pass in ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com