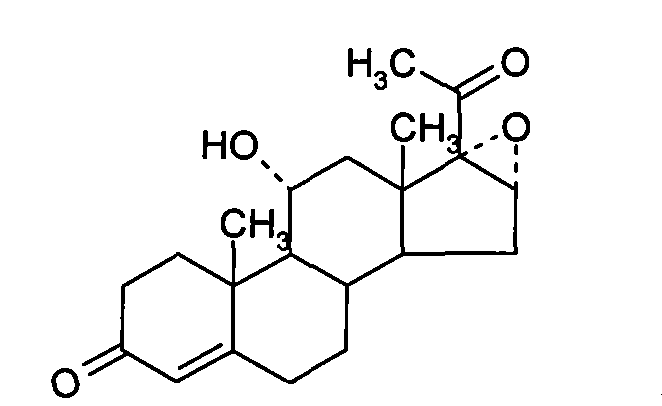
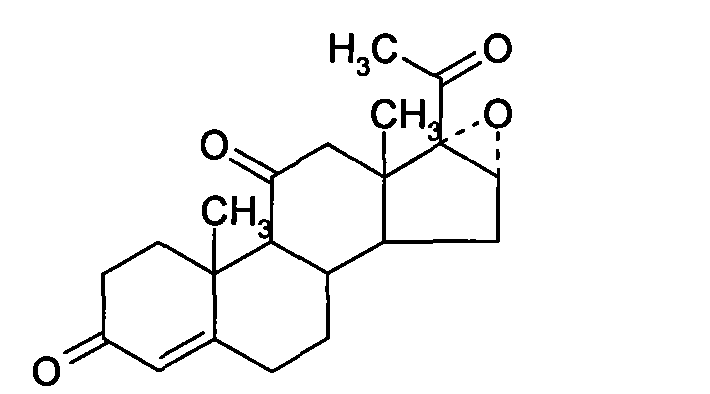
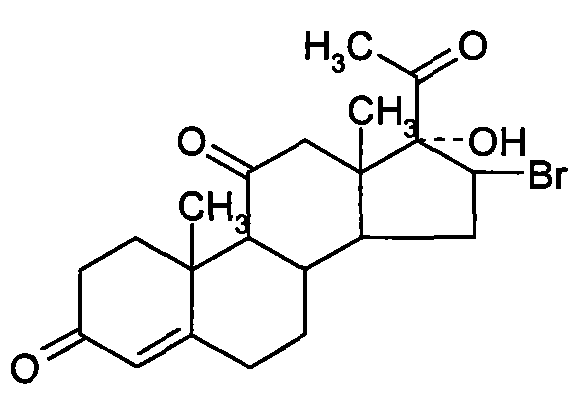
Chemical synthesis method of methylprednisolone
A methylprednisolone, chemical synthesis technology, applied in chemical instruments and methods, organic chemistry, steroids, etc., can solve the problems of product loss, low yield, difficulty in processing and purification, etc. The effect of less product, lower production cost and higher total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Platts Oxidation
[0034] Throw in 30 kg of mold oxide, add 10 kg of glacial acetic acid, stir and cool to 0°C, add 6 kg of manganese chloride aqueous solution (containing 0.1 parts by weight of manganese chloride and 0.1 parts by weight of water in the aqueous solution of manganese chloride), and cool to 10°C 18 kg of chromic anhydride aqueous solution was added dropwise (containing 0.3 parts by weight of chromic anhydride and 0.3 parts by weight of water in the aqueous solution of chromic anhydride), the temperature was controlled at 10°C, added dropwise for 3 hours, and reacted at 25°C for 6 hours after the dropwise addition, and added 900 kg of water, stirred for 1 hour for water analysis, centrifuged after water analysis, washed the filter cake until neutral, and dried at 70°C to obtain the Platinum compound.
[0035] (2) bromine reaction
[0036] Take 27 kg of Platinum, add 100 kg by weight of hydrogen bromide, stir and heat up to 30 ° C for 4 hours, add 1000...
Embodiment 2
[0057] (1) Platts Oxidation
[0058] Throw in 30 kg of mold oxide, add 15 kg of glacial acetic acid, stir and cool to 0°C, add 6 kg of manganese chloride aqueous solution (containing 0.1 parts by weight of manganese chloride and 0.1 parts by weight of water in the aqueous solution of manganese chloride), and cool to 10°C 18 kg of chromic anhydride aqueous solution was added dropwise (containing 0.3 parts by weight of chromic anhydride and 0.3 parts by weight of water in the aqueous chromic anhydride solution), the temperature was controlled at 15°C, added dropwise for 3 hours, and reacted at 20°C for 5 hours after the dropwise addition, and added 1000 kg of water, stirred for 1 hour for water analysis, centrifuged after water analysis, washed the filter cake until neutral, and dried at 70°C to obtain the Platinum compound.
[0059] (2) bromine reaction
[0060] Take 27 kilograms of Platinum, add 135 kilograms of hydrogen bromide, stir and heat up to 35 ° C for 4 hours, add 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com