Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41results about How to "Low therapeutic dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
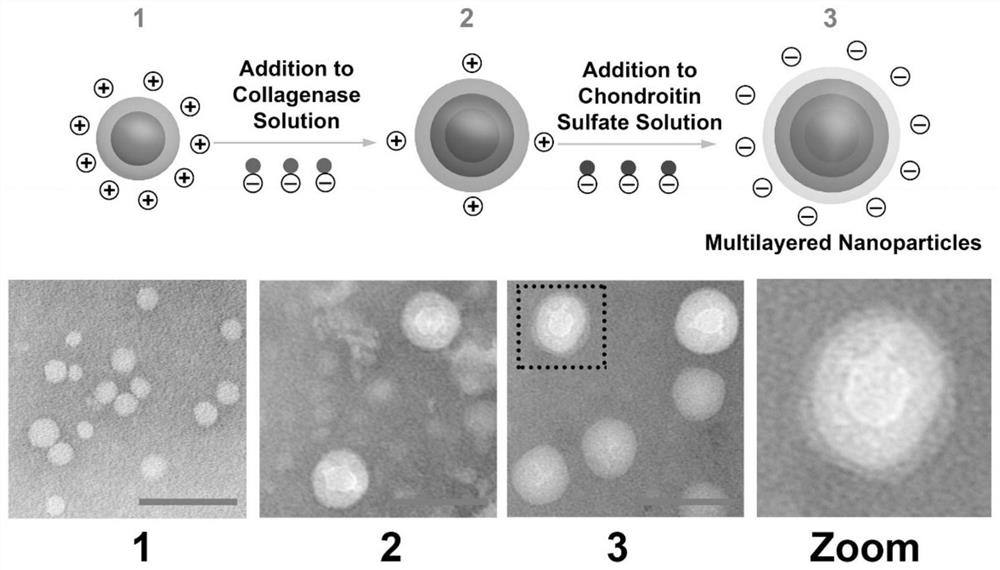
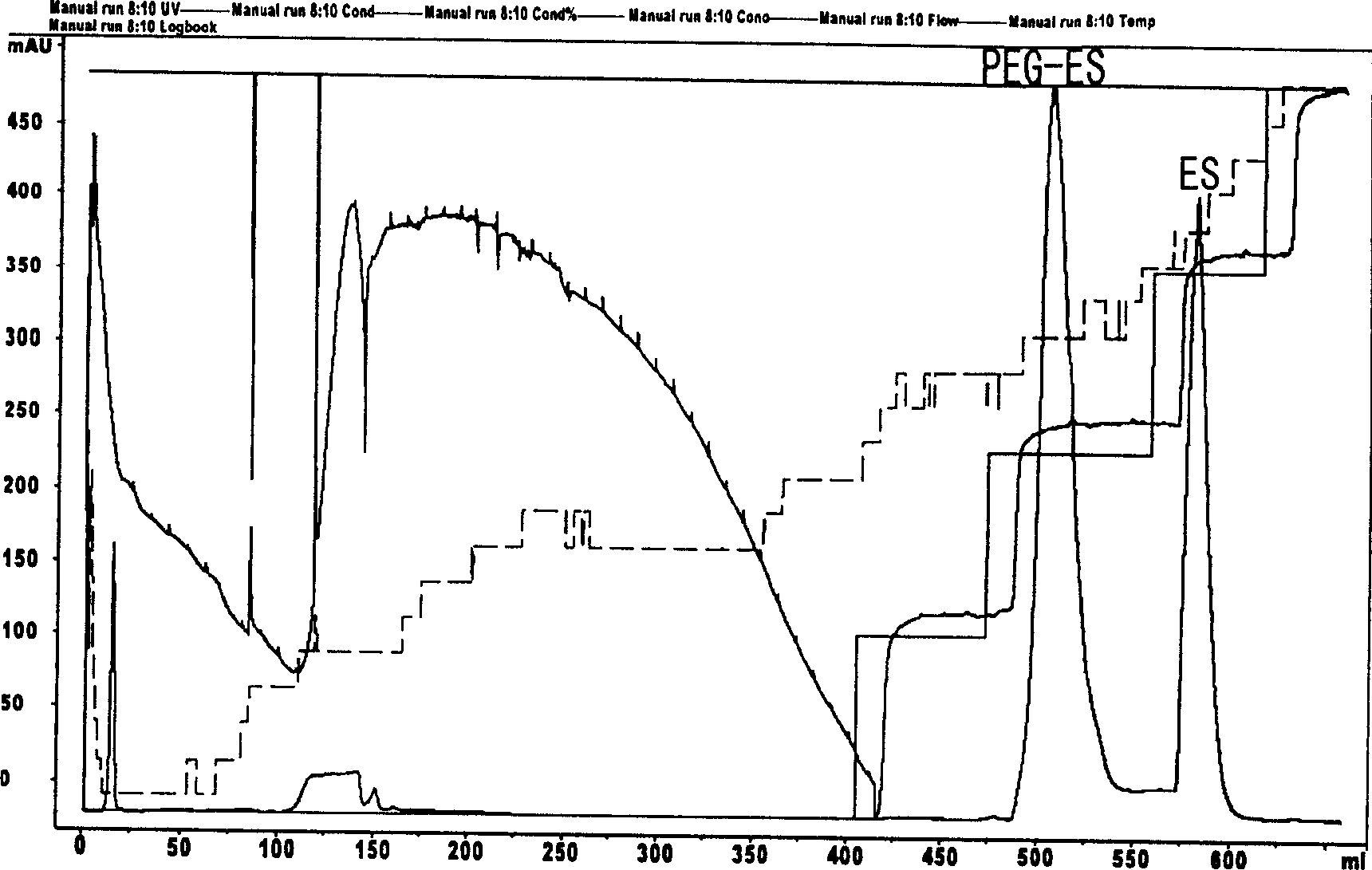
Chemical modification method of endostatin and its use
InactiveCN1891717ANot easy to hydrolyzeStable pHPeptide/protein ingredientsPeptide preparation methodsMonomethoxypolyethylene glycolChymotrypsin
The invention belongs to the field of bio-pharmaceutical products, which provides a chemical modification method for recombinate human rh-Endostatin ('rh-ES' is called for short) through the way of using mPEG-propionaldehyde to be combined on the fixed point of free amino of N-terminal amino acid of rh-ES. Mono-PEG-rhES is the output material, its purity meet the requirements of biological products. It plays a key role in the hydrolysis resistance of trypsin and chymotrypsin, it can be used as long-acting formulations for its stability for pH and temperature and there is a great perspective in the clinical treatment of cancer.
Owner:NANJING UNIV +1
Liposome of astragaloside IV and its medicinal preparation
InactiveCN101007013AImprove therapeutic indexLow therapeutic dosePowder deliveryOrganic active ingredientsAstragalosideMedicine
The invention relates to a astragaloside liposome and method for preparing same, wherein the liposome is prepared from astragaloside, phosphatide and lipophilic additives, the weight ratio of phosphatide and astragaloside is 0.5:1-100:1, the weight ratio of phosphatide and lipophilic additives is 1:1-50:1. The liposome can be used for preparing powdered inhalant, aerosols, sprays and oral mucosa doses.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Meloxicam tablets for dogs and cats and preparation method for meloxicam tablets
InactiveCN102525974AReduce stimulationGood dissolution effectOrganic active ingredientsAntipyreticCross-linked polyethyleneCitrate sodium
The invention relates to the field of medicine, and discloses meloxicam tablets for dogs and cats and a preparation method for the meloxicam tablets. The preparation method mainly comprises: a mixing step, a granulation step, a drying step, a granulation straightening step, a granule mixing step and a tabletting step. The meloxicam tablets consist of the following components in percentage by mass: 0.1 to 0.6 percent of meloxicam, 50 to 70 percent of lactose, 20 to 40 percent of microcrystalline cellulose, 3 to 5 percent of hydroxypropyl cellulose, 0.4 to 0.6 percent of sodium citrate, 1 to 5 percent of cross-linked polyvinylpyrrolidone, 4 to 8 percent of polyvinylpyrrolidone (PVP) K30, 0.2 to 0.8 percent of aspartame and 0.60 to 2 percent of magnesium stearate. The meloxicam tablets have small irritation on the intestines and stomach of the dogs and the cats, and are favorable for the absorption of active ingredients.
Owner:NANJING SBEED BIOTECH
Isomorellic acid polylactic acid nano particle preparation and preparing method thereof
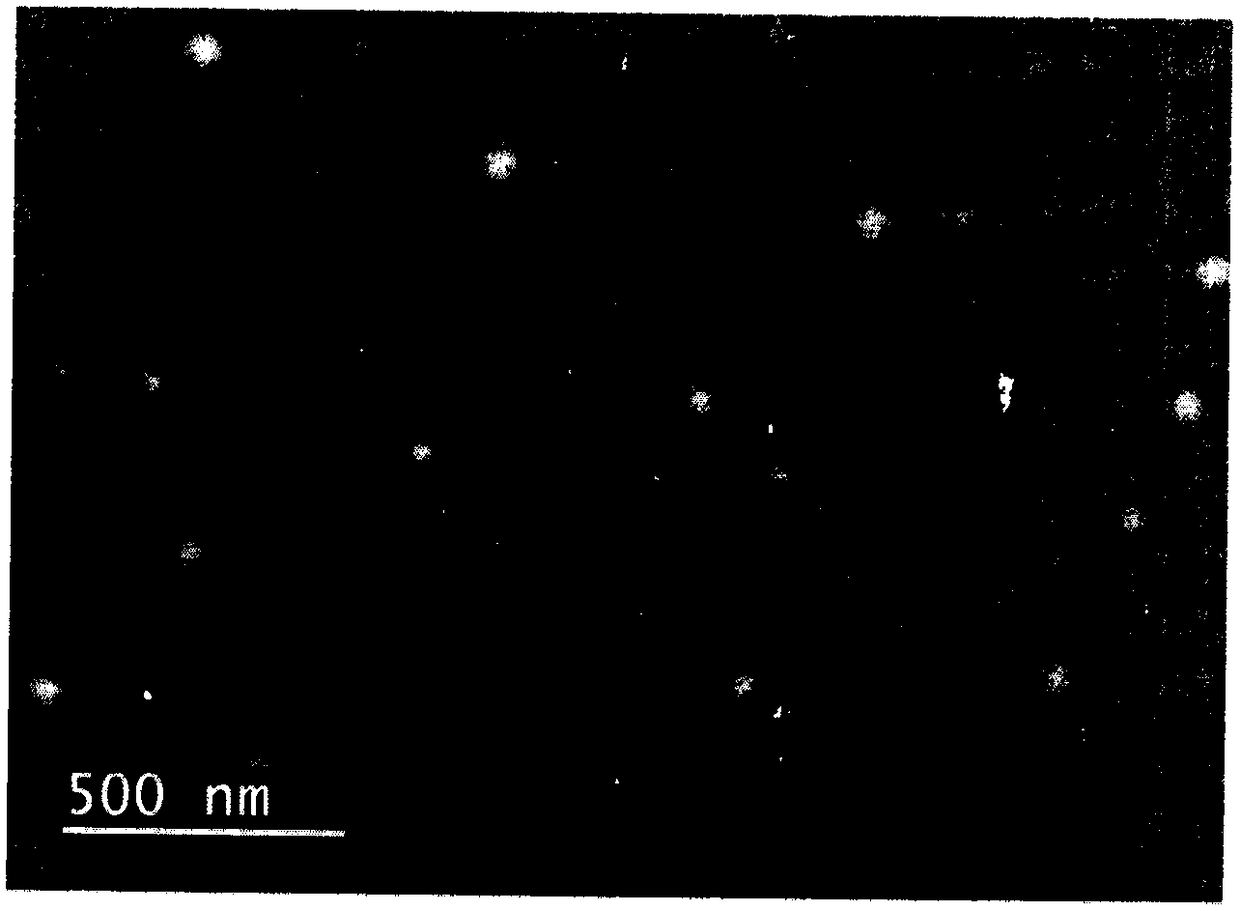
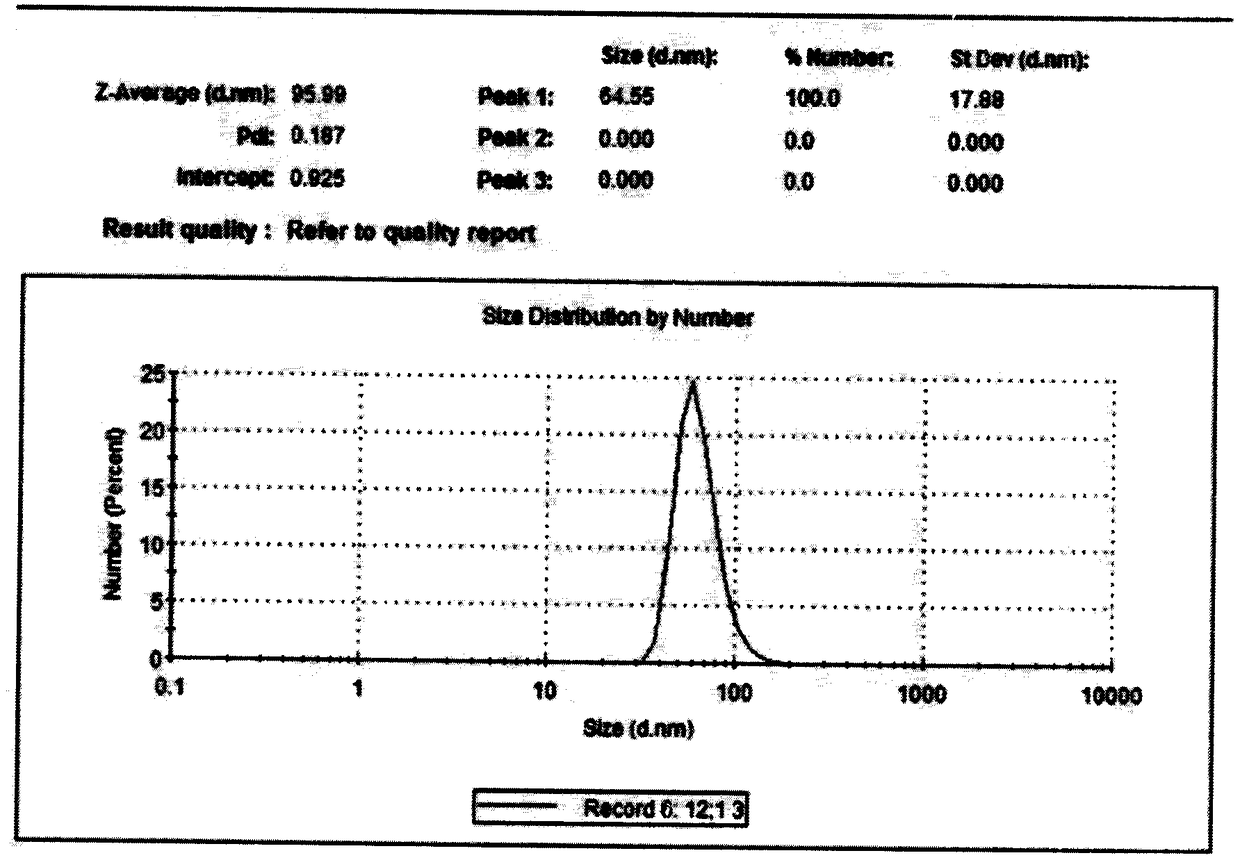
InactiveCN101229130ANanoparticles with low drug loadingPossesses liver passive targeting propertiesOrganic active ingredientsPowder deliverySide effectTherapeutic effect
The invention discloses a gamboges acid nanometer particle preparation and is prepared by the components of raw material gamboges acid 0.05 to 1.5 percent, polylactic acid 0.05 to 1.5 percent, surfactant 0.1 to 1.5 percent, organic solvent 33 to 50 percent and the allowance of water with the following steps: dissolving the polylactic acid into the organic solvent and dispersing the gamboges acid in the mixed solution of the polylactic acid and the organic solvent; the preparation is obtained by adding the mixed solution with the gamboges acid into water phase with the surfactant, stirring magnetically for 4 hours, vacuum distillation and filtering with a 0.22Mum micro-porous filtering film after the organic solvent and part of water are distillated. The drug with a clear system, the particle size between 10 to 100nm and good dispersion and stability can significantly solubilize drugs, has a targeting effect for reticuloendothelial cells and concentrate at lesion sites, thus improving the curing effect of the drug and reduces the side effect of the drug. Additionally, the drug has a slowly releasing effect, can maintain constant plasma concentration or pharmacologic effect for a long time and improve drug bioavailability.
Owner:NORTHWEST A & F UNIV
Docetaxel long-circulating liposome and preparation method thereof
InactiveCN101884616AImproved disposal in the bodyImprove disposalOrganic active ingredientsPharmaceutical non-active ingredientsWater bathsDocetaxel-PNP
The invention aims to provide a method for preparing a docetaxel long-circulating liposome. The method comprises the following steps of: dissolving docetaxel and carrier materials such as phospholipid, cholesterol and the like together in an organic solvent; forming a film through rotary evaporation in a 40 to 70 DEG C water bath; adding buffer solution which contains a surfactant and performing refrigeration at the temperature of below 4 DEG C for 10 to 60 minutes to fully hydrate the film; and then mixing or stirring to obtain the docetaxel liposome. The liposome of 70 to 500nm is prepared from the docetaxel and can be prepared into docetaxel long-circulating liposome frozen powder for injection by lyophilization.
Owner:SHANGHAI GENE CELL BIO MEDICAL TECH +1
Preparation method of small unilamellar vesicle liposome of ivermectin
InactiveCN101664389AEvenly distributedImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolEvaporation
The invention discloses a preparation method of small unilamellar vesicle liposome of ivermectin, and relates to a preparation method of ivermectin liposome, which solves the problems that the existing ivermectin preparation has short effective date, and needs to deliver drug repeatedly and the sustained release macro-pill of the ivermectin has high toxicity. The method comprises the following steps of: adding raw ivermectin powder in organic solution of soya bean lecithin and cholesterol, then carrying out pressure reduction, rotation and evaporation to the obtained injectable suspension tilla layer of thin film is formed at the inner wall of a reactor, then dissolving the thin film in PBS solution, adding glass beads into a rotary-type oscillator, shaking to wash the film for 1 hour, then standing for 1 hour, and carrying out ultrasonic oscillation so as to obtain the small unilamellar vesicle liposome of ivermectin. The liposome obtained by the preparation method has similar structure as a biofilm, can be biodegraded in the organism, does not generate toxic substances, and can be slowly and continuously released as embedded drug in a storage mode, thus being capable of prolonging effective horizontal period of the drug and reducing drug delivery frequency; and the packet rate of the small unilamellar vesicle liposome of ivermectin is 89.5%.
Owner:INST OF ANIMAL SCI & VETERINARY TIBET ACADEMY OF AGRI & ANIMAL HUSBANDRY SCI
Modified dosage forms of tacrolimus
InactiveCN101652141AImprove complianceLower metabolismPowder deliveryPill deliveryTime rangeOral medication
The present invention provides a modified release dosage form of tacrolimus that releases two or more amount of tacrolimus upon oral administration, the first amount of tacrolimus releases from the immediate release dosage unit substantially immediately within 0-2 hours followed by a time interval ranging from about 1-10 hours during which substantially no amount of tacrolimus is released from thedosage form, after which a second amount of tacrolimus is released wherein said second amount is released from the delayed release dosage unit either immediately e.g. within 0-2 hours or over a period of time ranging from about 2-12 hours from its initial release from the delayed release dosage unit. The dosage form may further comprise additional amount of tacrolimus to provide additional pulseof tacrolimus. The dosage forms of tacrolimus exhibit improved bioavailability and reduced flux or fluctuation over existing composition of tacrolimus. A method of preparing the dosage forms is also described.
Owner:PANACEA BIOTEC
Preparing method of compound brucea javanicaseed oil liposome freeze-dried powder
InactiveCN103599078AInhibit synthesisLow therapeutic doseAmphibian material medical ingredientsPowder deliveryMicropore FilterPenicillin
The invention provides a preparing method of a compound brucea javanicaseed oil liposome freeze-dried powder, and belongs to the technical field of traditional Chinese medicine preparation. The preparing method is characterized by comprising the following steps: 1) weighing soybean phospholipids, cholesterol, vitamin E and brucea javanicaseed oil, dissolving by ethanol, and taking as an oil phase; 2) measuring a toad venom aqueous extract, adding mannitol and sucrose, and adding distilled water to as an aqueous phase; 3) injecting the oil phase into the aqueous phase, and extruding by a micropore filter membrane to obtain a suspension; 4) filling a penicillin bottle with the suspension, carrying out freeze drying, and thus obtaining the compound brucea javanicaseed oil liposome freeze-dried powder. The preparation method of the compound brucea javanicaseed oil liposome freeze-dried powder adopts the brucea javanicaseed oil and toad venom for combined application, the average encapsulation rate of the prepared liposome freeze-dried powder is 91.90%, the average content of indole alkaloids is 5.19*10<-3> mg.g<-1>, the average content of oleic acid is 79.08 mg.g<-1>, the average content of linoleic acid is 50.41 mg.g<-1>, the antitumor effect is good, and toxic and side effects are small.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
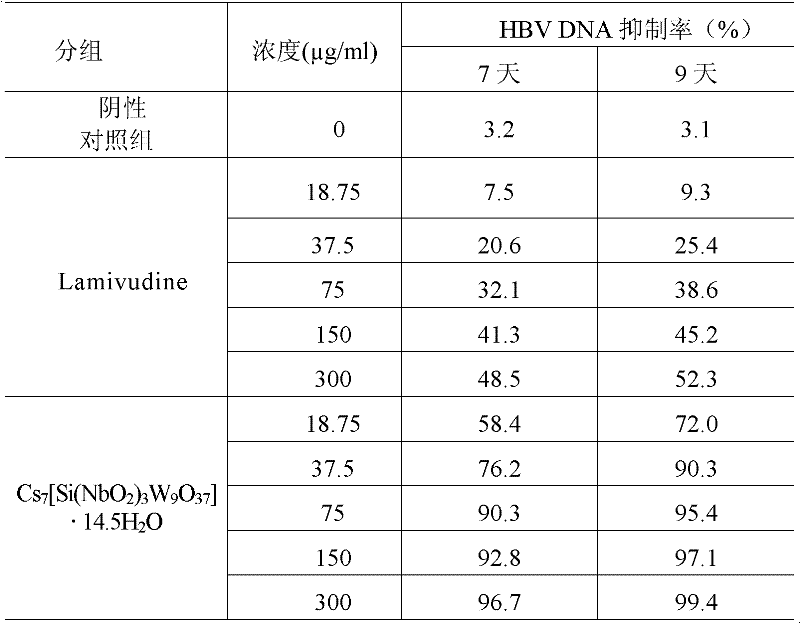
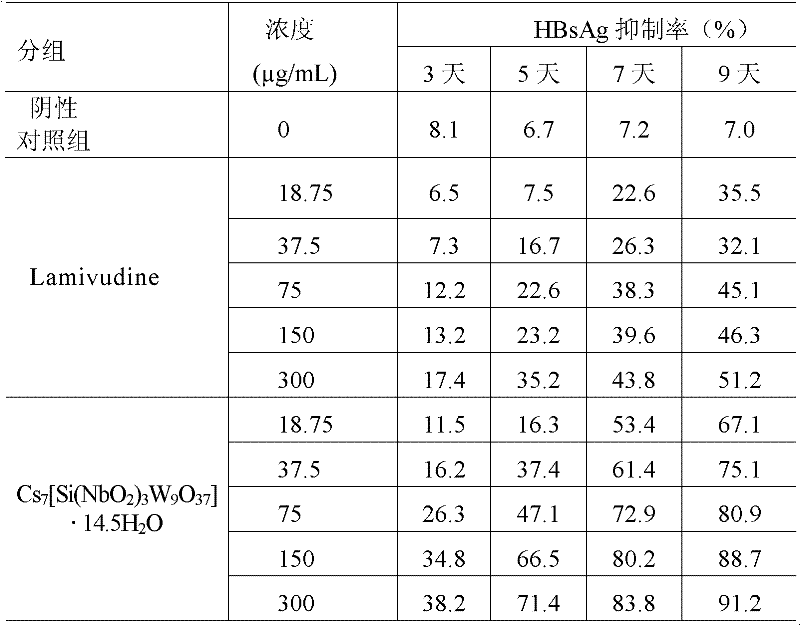
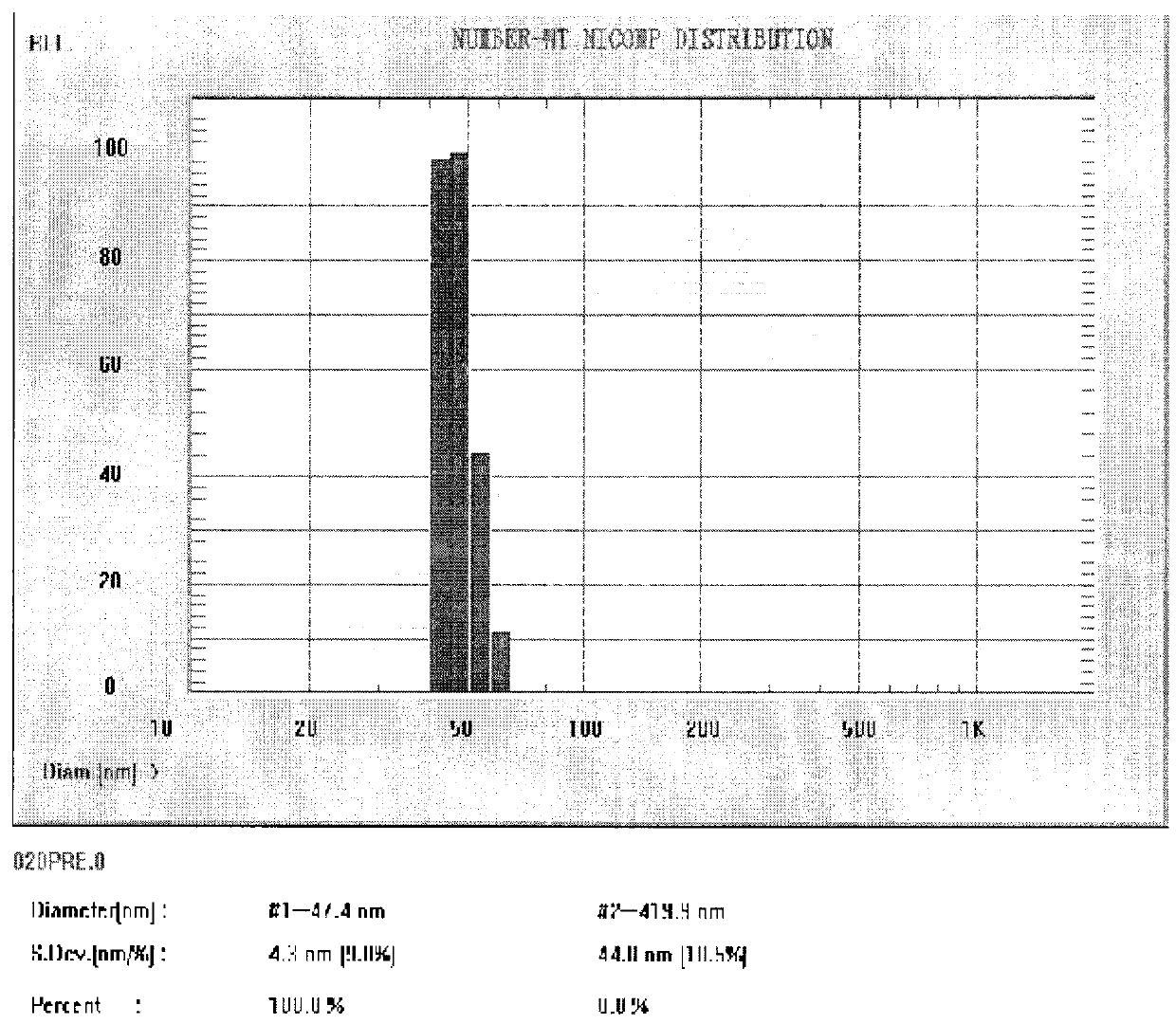
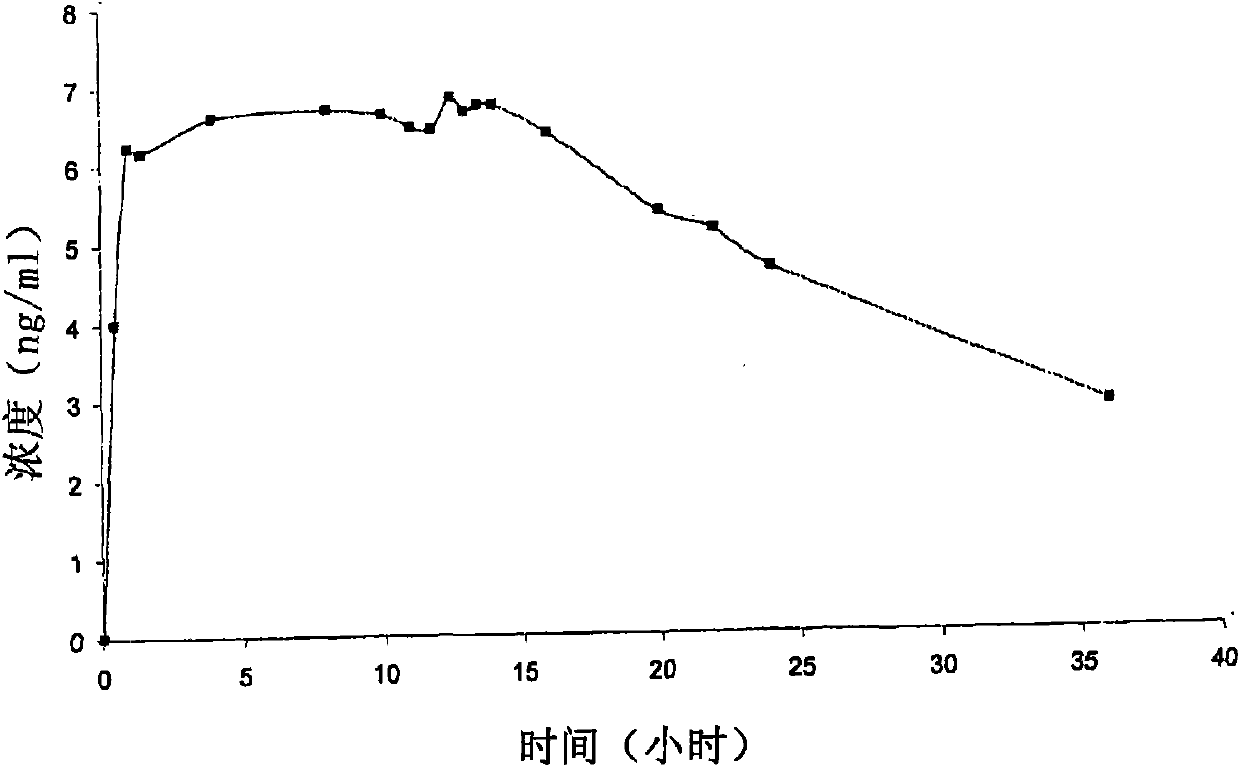
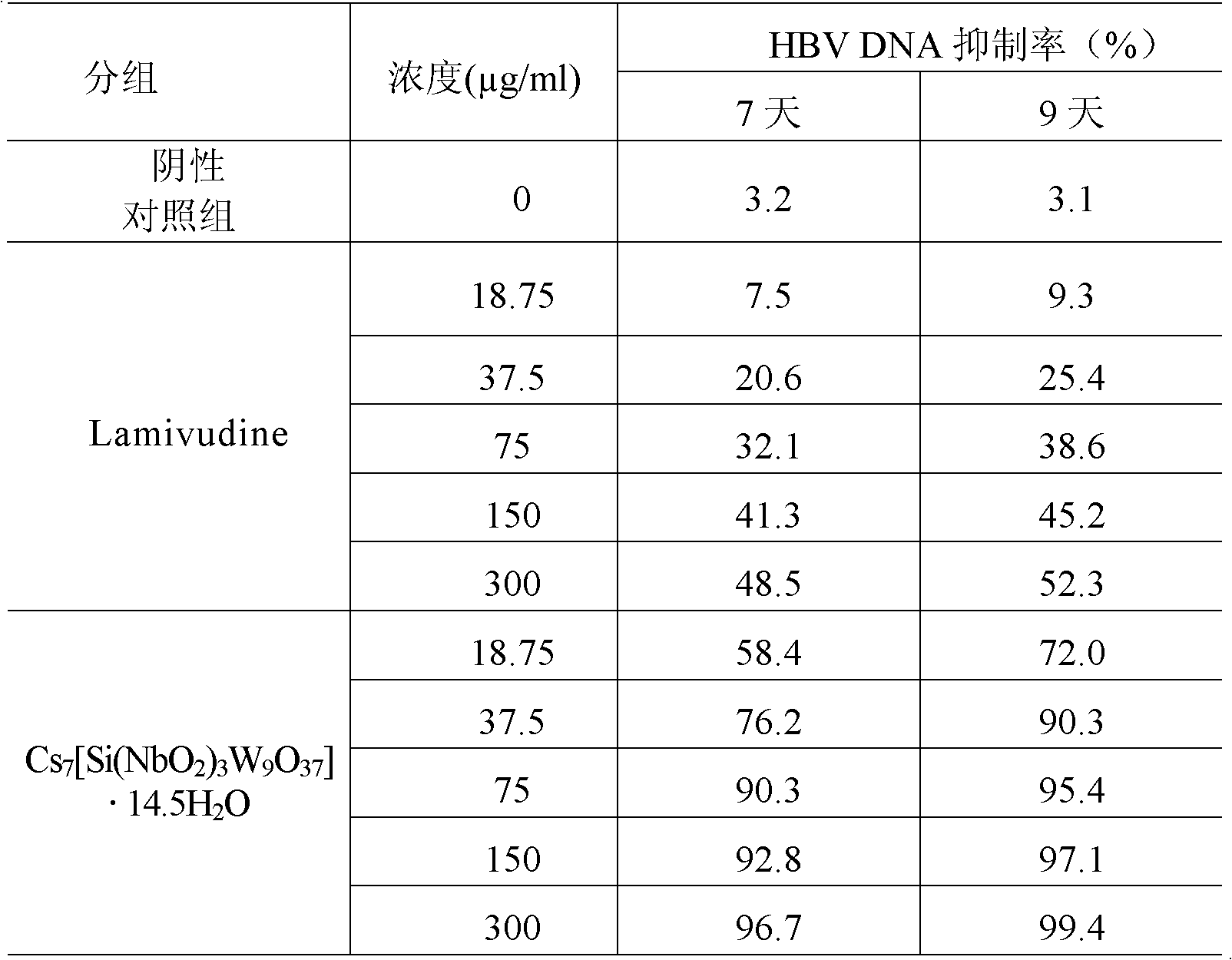
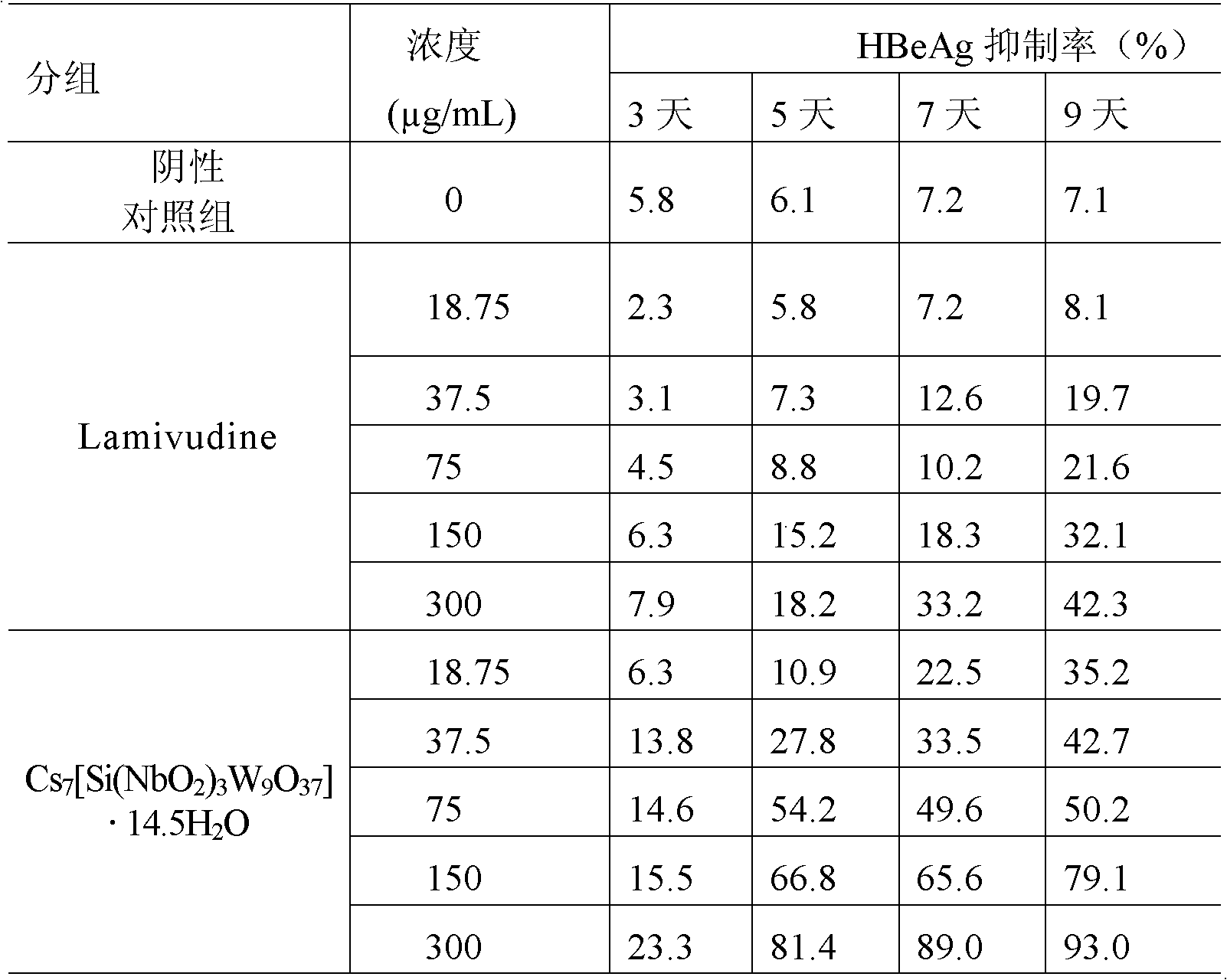
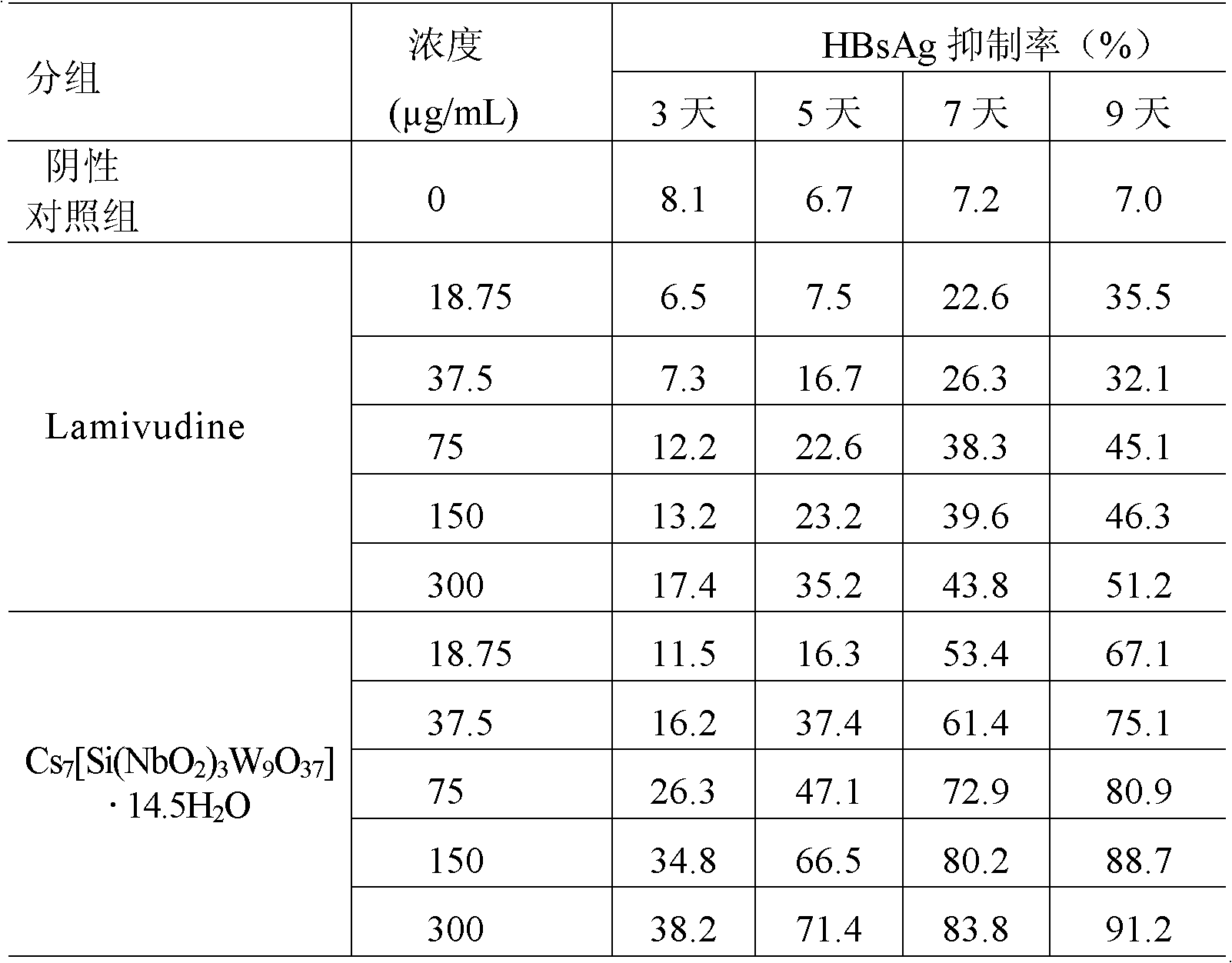
Niobium tungstate compound for resisting hepatitis virus
InactiveCN101844811ASynthetic raw materials are readily availableLow priceHeavy metal active ingredientsOrganic chemistryTungstateNiobium
The invention provides a niobium tungstate compound for resisting hepatitis virus, which has the function of resisting the hepatitis virus and the chemical formula of AmBr (X (NbO2) 3W9O37). nH2O, wherein A is alkali metal ion Li, Na, K or Cs; B is hydrogen cation H+, ammonium ion NH4+ or amino acid cation; X is Si, Ge or P; and m=0-7, r=0-7 and n=0-20. The niobium tungstate compound is taken as active ingredient which is assisted by carrier or auxiliary material that is pharmaceutically acceptable, thus being used for preparing oral preparation or injection medicine applied to treating hepatitis. Compared with the existing medicine which is applied to treating hepatitis clinically, the niobium tungstate compound has the advantages of better liver target, low effective therapeutic dose, higher therapeutic index and lower toxicity, thus being an antiviral compound which has higher efficiency and is safer.
Owner:JILIN UNIV
Application of 2-phenylpyrazole [1,5-a] pyrimidine compounds serving as tumor drug resistance reversal agent
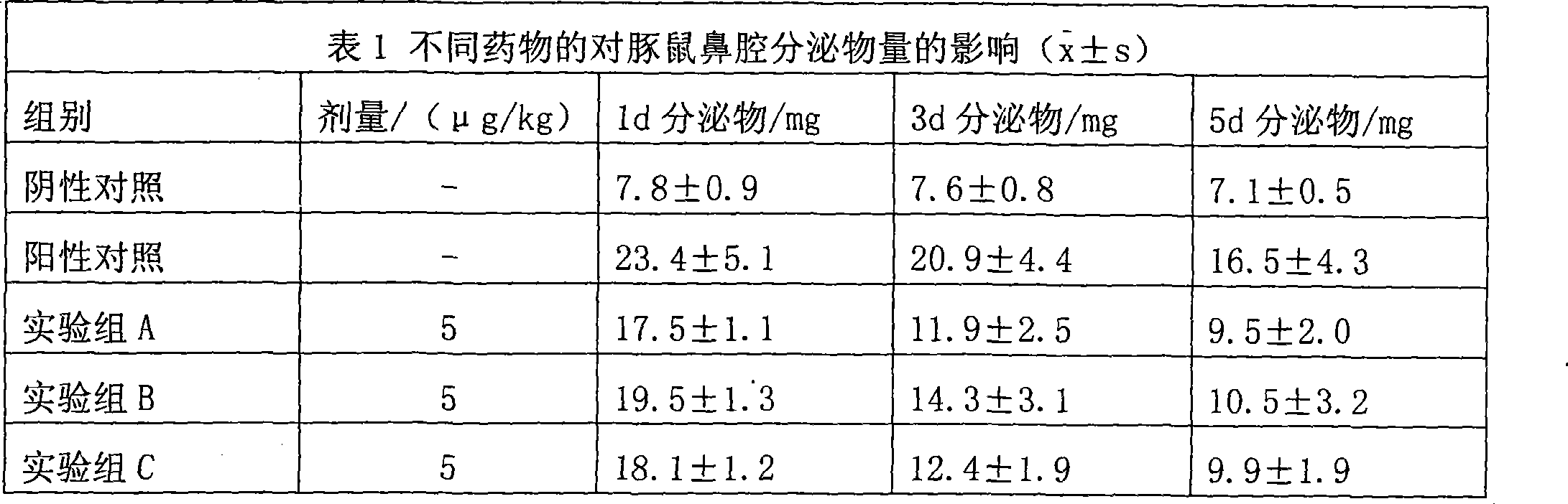
ActiveCN111388477ALow therapeutic doseEasy to useOrganic active ingredientsAntineoplastic agentsPyrimidineMolecular biology
The invention particularly relates to application of 2-phenylpyrazole [1,5-a] pyrimidine compounds serving as a tumor drug resistance reversal agent. Aiming at the problem of cancer chemotherapy resistance, the invention provides compounds having an inhibiting effect on the ATP-binding cassette sub-family G member 2 (ABCG2), and the compounds are used for improving the drug resistance of tumor cells. According to the application, a series of 2-phenylpyrazole [1,5-a] pyrimidine compounds are obtained by study, so that the ABCG2 in drug resistance-type tumor cells can be in a resting state, pumping out of a substrate is blocked, and the drug resistance of tumors is reversed, and the therapeutic dose is safe and non-toxic. Based on the result of the study, the compounds can be combined with other ABCG2 drug resistance related antitumor drugs for treatment; and the 2-phenylpyrazole [1,5-a] pyrimidine compounds have good clinical medication significance.
Owner:SHANDONG NORMAL UNIV
Nanoparticulate leukotriene receptor antagonist/corticosteroid formulations
InactiveCN101175480AImprove convenienceImprove compliancePowder deliverySenses disorderNasal cavityPediatric patient
Nanoparticulate compositions containing corticosteroids and leukotriene receptor antagonists are described. The composition is used for prevention and long-term treatment of asthma in adults and children, and for relieving symptoms of allergic conjunctivitis and seasonal allergic rhinitis in adults and children. Combining a leukotriene receptor antagonist in the particle size range of less than 2000nm with a corticosteroid in one formulation resulted in increased potency. Additionally, patient compliance is enhanced since only one dosage form is required. Furthermore, topical administration of leukotriene receptor antagonists results in lower hepatotoxicity, since the liver will be exposed to a smaller amount of drug than would be the case after oral administration. The pharmaceutical compositions of the present invention may be formulated for inhalation, nasal or ophthalmic use.
Owner:ELAN PHRMA INT LTD
Uses of methylprednisolone and derivatives thereof in preparing medicament for treating allergic rhinitis
ActiveCN101347436BDecreased systemic effectsLow therapeutic doseOrganic active ingredientsAerosol deliveryNasal cavityAdditive ingredient
A pharmaceutical composition for treating allergic rhinitis comprises one or a plurality of kinds from methylprednisolone taken as active ingredient or pharmaceutically acceptable salt thereof or esterified matters thereof, and a pharmaceutical composition consisting of one or a plurality of inactive ingredients applicable to local action inside nasal cavity.
Owner:天津药业集团有限公司
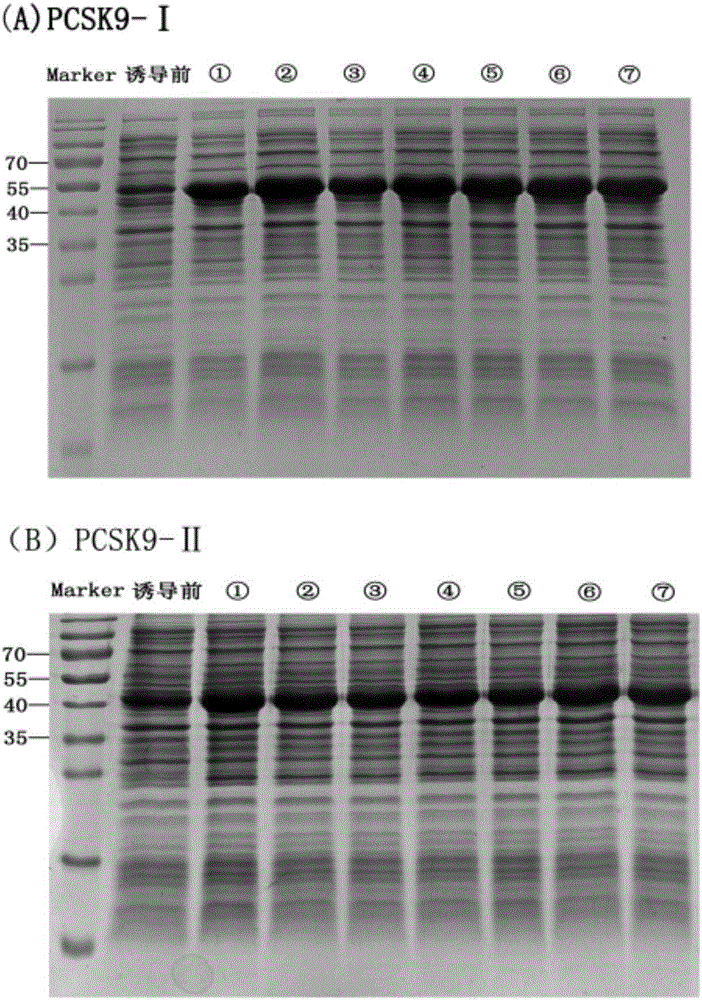
Anti-hyperlipidemia protein vaccine aiming at PCSK9 (Proprotein convertase subtilisin/kexin type 9)
InactiveCN106822881AAvoid degradationAvoid toleranceMetabolism disorderVertebrate antigen ingredientsKexinSubtilisin
The invention belongs to the field of biomedicines and relates to an anti-hyperlipidemia protein vaccine aiming at PCSK9 (Proprotein convertase subtilisin / kexin type 9). Aiming at solving the technical problems, the anti-hyperlipidemia protein vaccine which has good performances and takes the PCSK9 as a target is researched and developed. The protein vaccine aiming at the PCSK9, which is prepared by the invention, can be used for remarkably lowering the level of blood lipid of serum and has remarkable immunogenicity and good safety; a preparation method is simple and feasible and is low in cost. The protein vaccine aiming at the PCSK9 can be used for preventing and treating hyperlipidemia diseases and can realize treatment effect without the need of completely blocking the PCSK9; a condition that individuals is intolerant to complete blocking of the PCSK9 is avoided and the anti-hyperlipidemia protein vaccine has a better application prospect.
Owner:SICHUAN UNIV
Hydroxyl camptothecin flexible liposome and preparation method thereof
InactiveCN102397254AGood curative effectLow therapeutic doseOrganic active ingredientsPharmaceutical non-active ingredientsWater bathsCholesterol
The invention discloses a hydroxyl camptothecin flexible liposome and a preparation method thereof. The method comprises the following steps of: dissolving hydroxyl camptothecin and carrier materials, such as phospholipid, cholesterol and the like, in an organic solvent; forming the mixture into a membrane in water bath through rotary evaporation; and adding buffer solution which contains chitosan into the membrane to form the hydroxyl camptothecin flexible liposome. The hydroxyl camptothecin flexible liposome is relatively lower in toxicity, relatively longer in residence time in a body, high in stability in a solution phase and smalllow in particle size, and the particle size of the liposome is not obviously changed after the liposome is lyophilized.
Owner:GUANGXI ZHUANGDU BIO TECH
Tumor ECM degrading and/or inhibiting agent and complete set kit and application thereof
PendingCN110404077AAvoid degradationSuppress generationAntineoplastic agentsPharmaceutical active ingredientsSide effectCell-Extracellular Matrix
The invention discloses a tumor ECM degrading and / or inhibiting agent and a complete set kit and application thereof. The tumor ECM degrading and / or inhibiting agent comprises at least two kinds of ananti-hyaluronidase agent, an anti-collagen agent, and an anti-tissue agent. The tumor ECM degrading and / or inhibiting agent can effectively degrade or restrain generation of a tumor extracellular matrix in a broadspectrum manner, and the tumor penetrability is improved, so that drugs can effectively enter tumors to be in contact with and kill tumor cells, and improve the medication effect. The combination manner can be applied in an intratumoral injection manner, so that the applying concentration of single components can be reduced, and the side effect is low. The complete set kit can increase the medication effect, the medicament dose can be reduced, and dosage required for achieving the same effect by an antitumor intratumoral injection manner is lower than that used for venous or other nonintratumoral injection, the antitumor dosage is reduced, and besides, the antitumous effects are increased.
Owner:SUZHOU KANGJU BIOTECHNOLOGY CO LTD
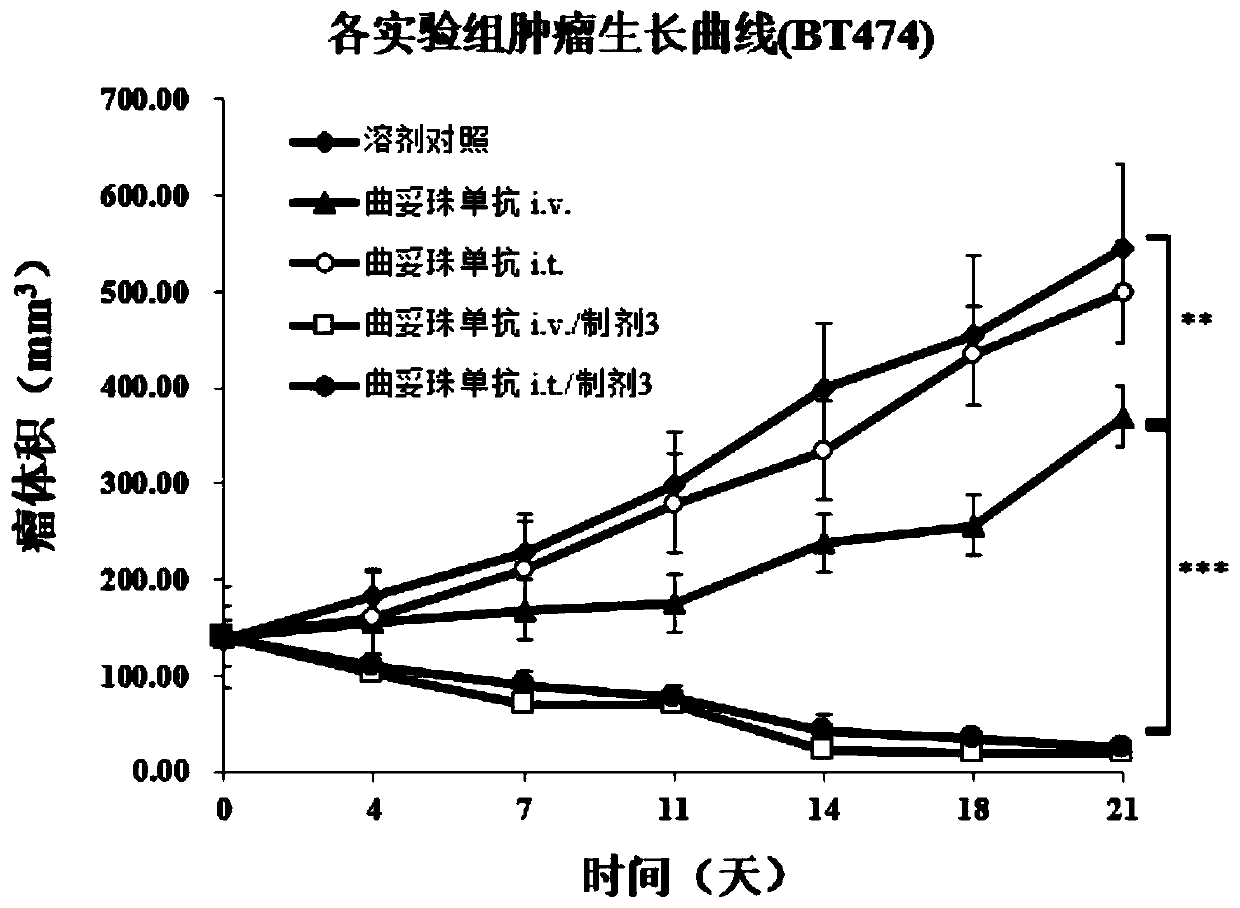
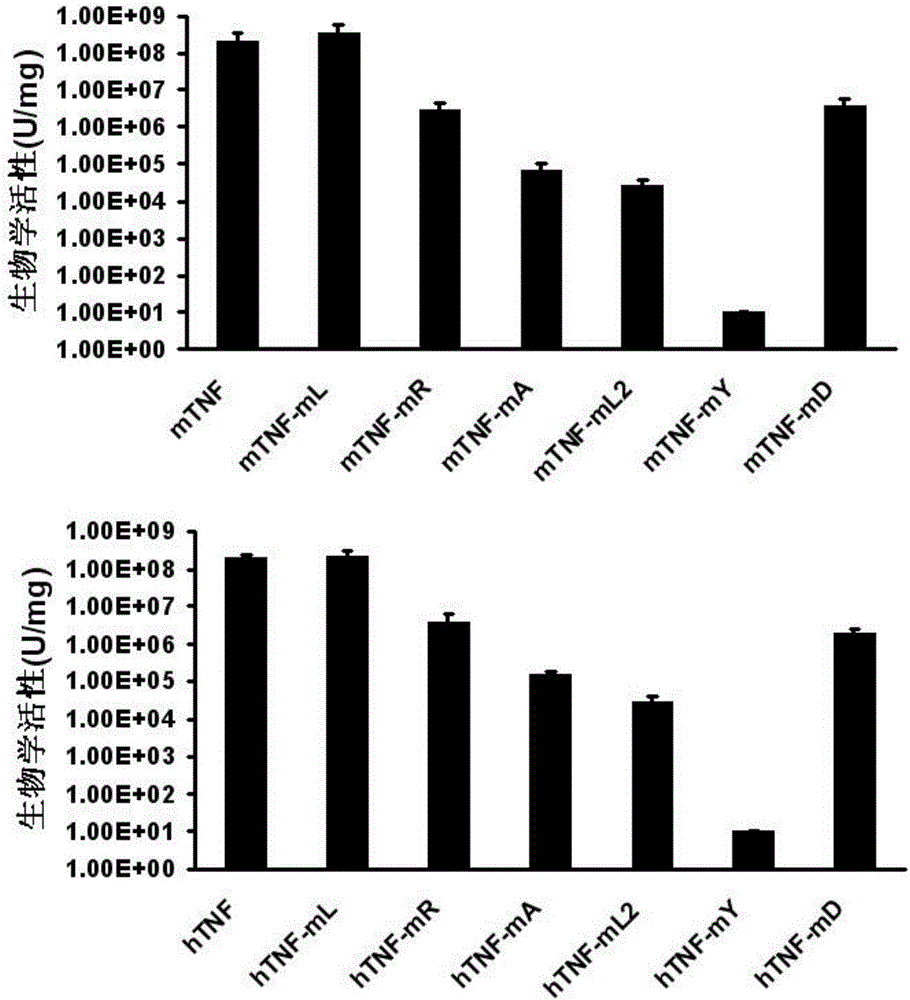
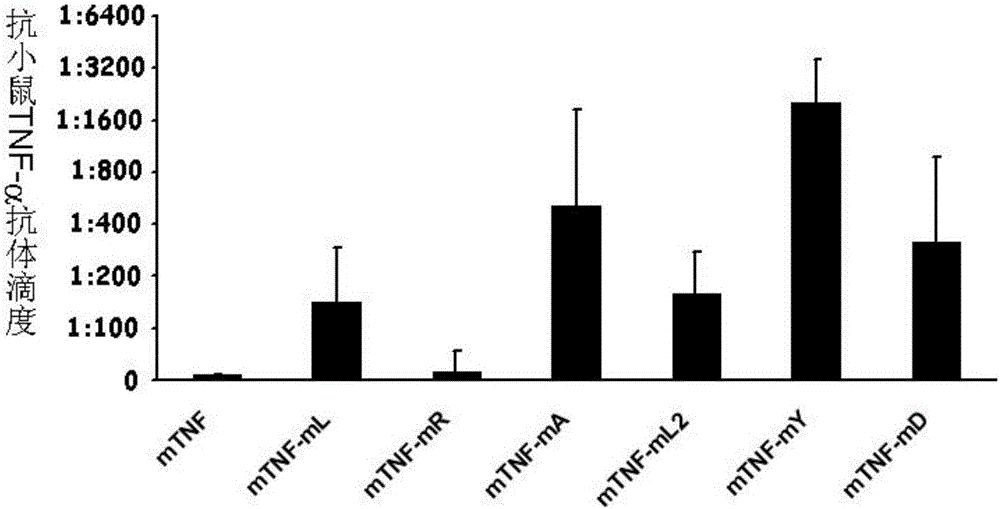
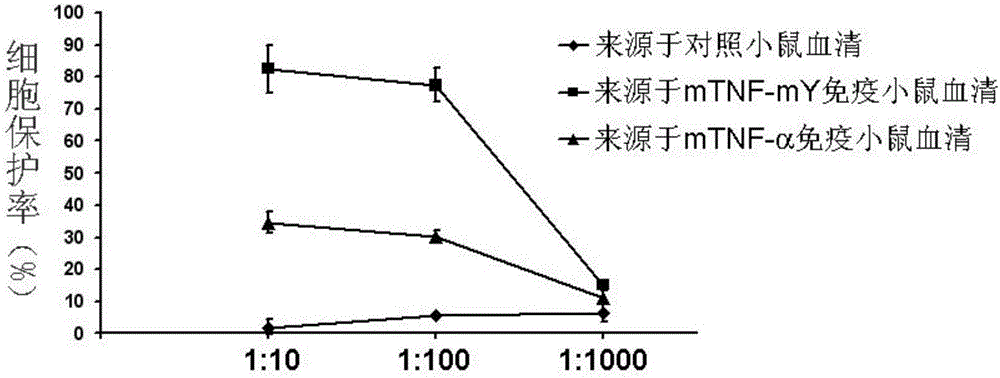
Protein vaccine aiming at tumor necrosis factor alpha and applications of protein vaccine
ActiveCN106540252AInhibit biological activityInhibit the inflammatory responseAntipyreticAntibody mimetics/scaffoldsImmunologic disordersMutated protein
The invention belongs to the field of biological medicines, and relates to a mutant protein vaccine aiming at tumor necrosis factor alpha (TNF-alpha) and applications of the mutant protein vaccine. The invention aims at providing the protein vaccine which is good in property and takes TNF-alpha as the target spot. According to the scheme, by immunizing the TNF-alpha mutant protein vaccine, the biological activity of the TNF-alpha mutant protein vaccine is inhibited, the cross immunologic reaction is generated in vivo, the biological functions of TNF-alpha are neutralized, and the autoimmune diseases and inflammatory diseases are inhibited. The protein vaccine aiming at TNF-alpha can be used for treating the autoimmune diseases and the inflammatory diseases and preventing the relapse of the autoimmune diseases and the inflammatory diseases.
Owner:SICHUAN UNIV
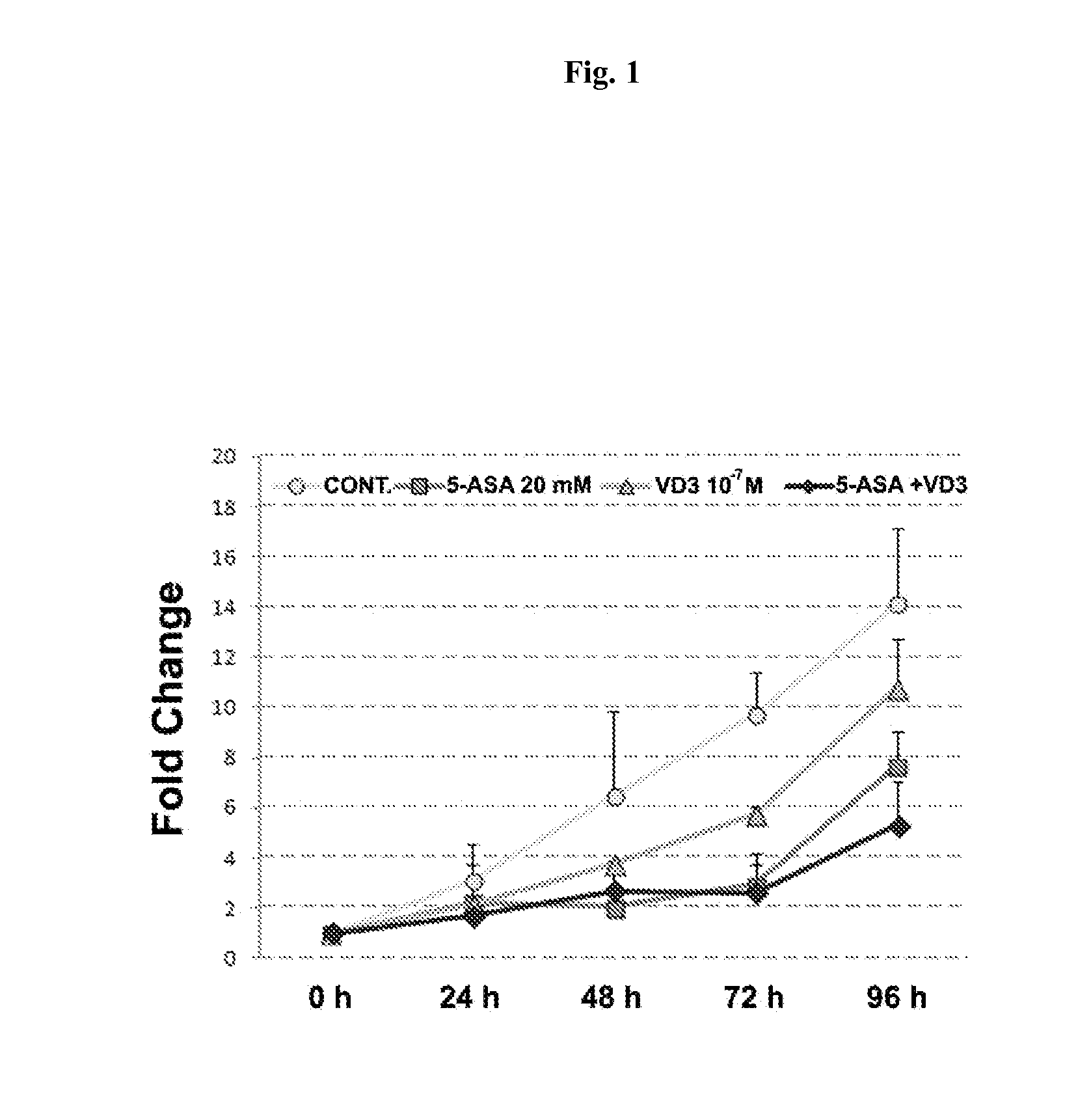
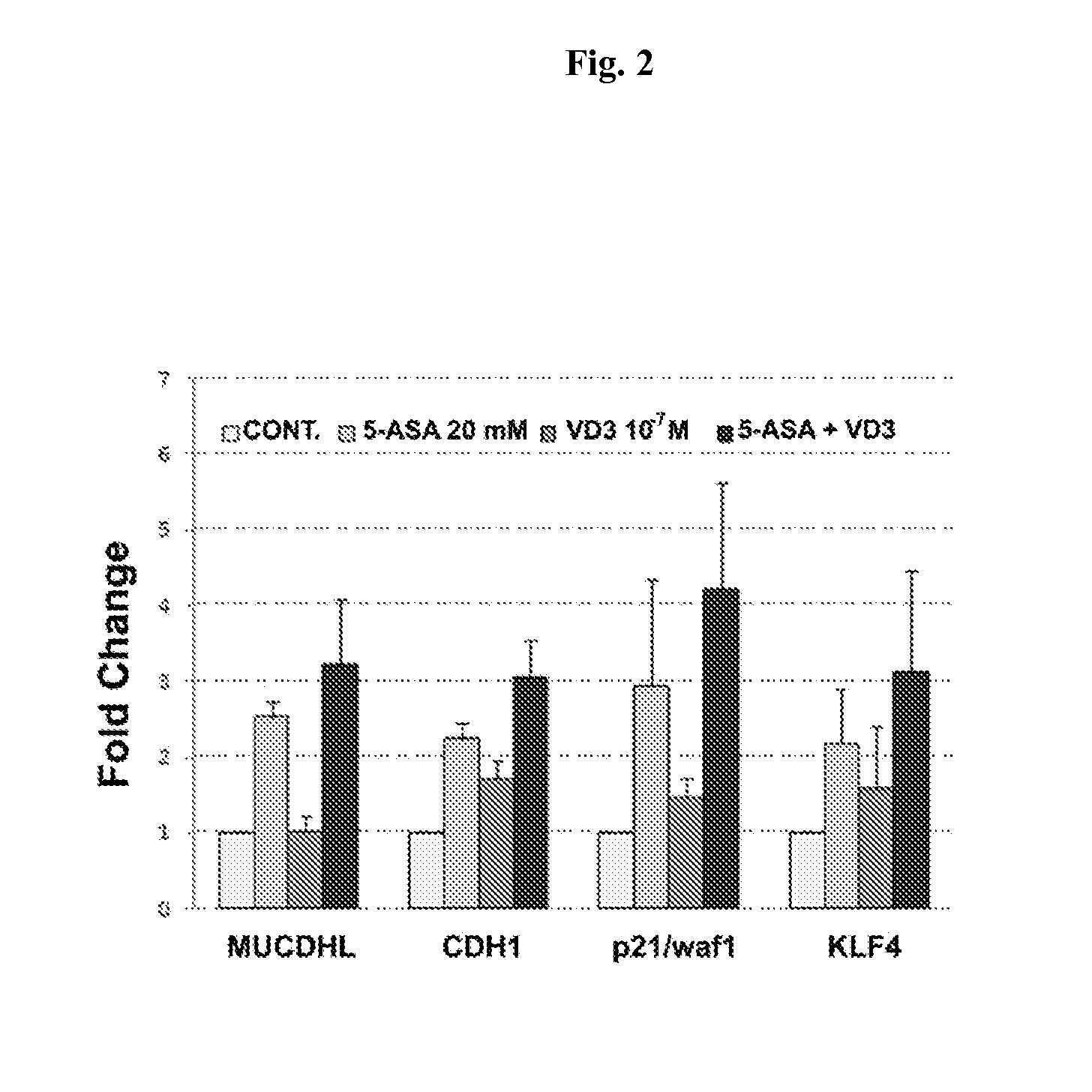
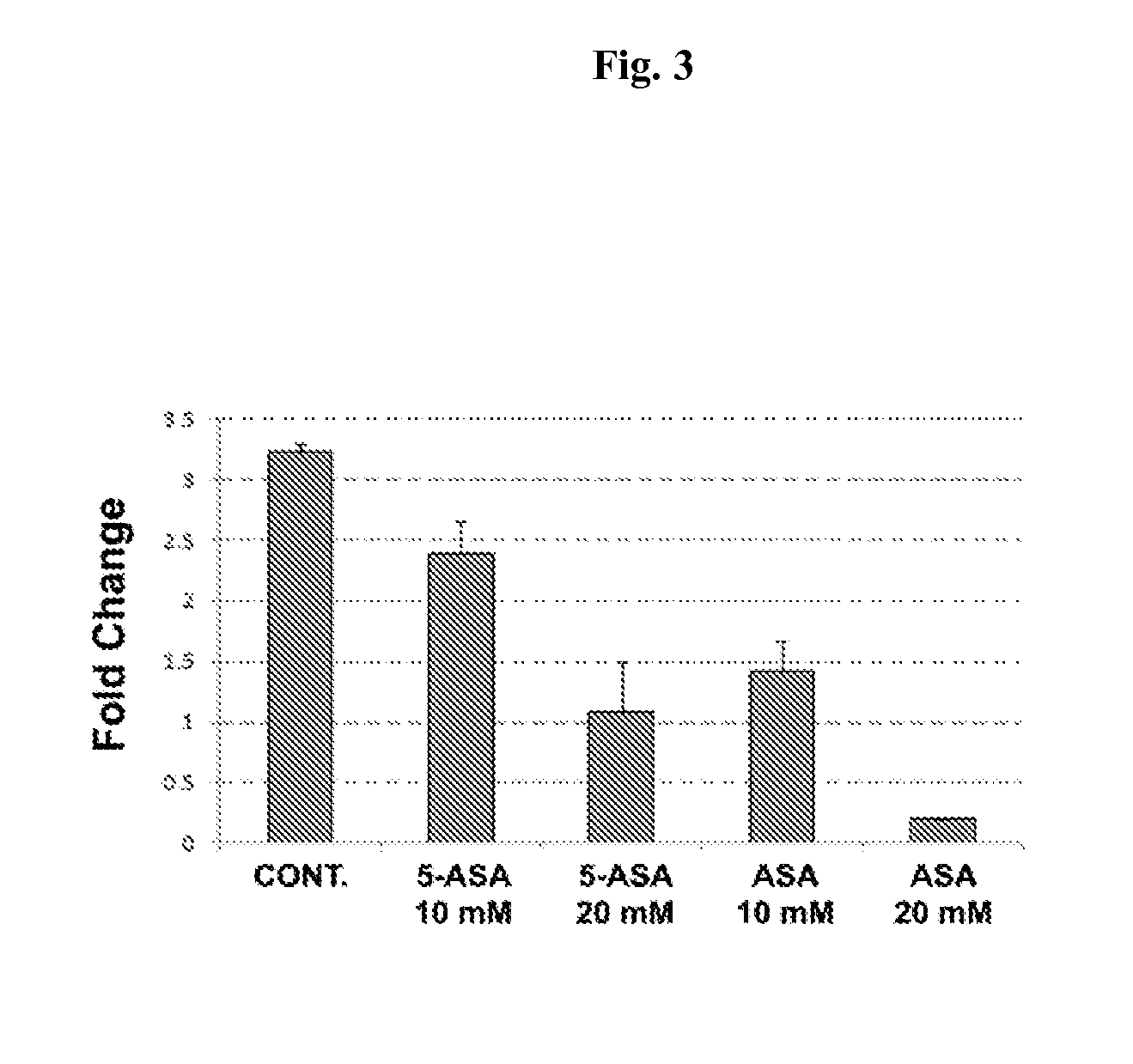
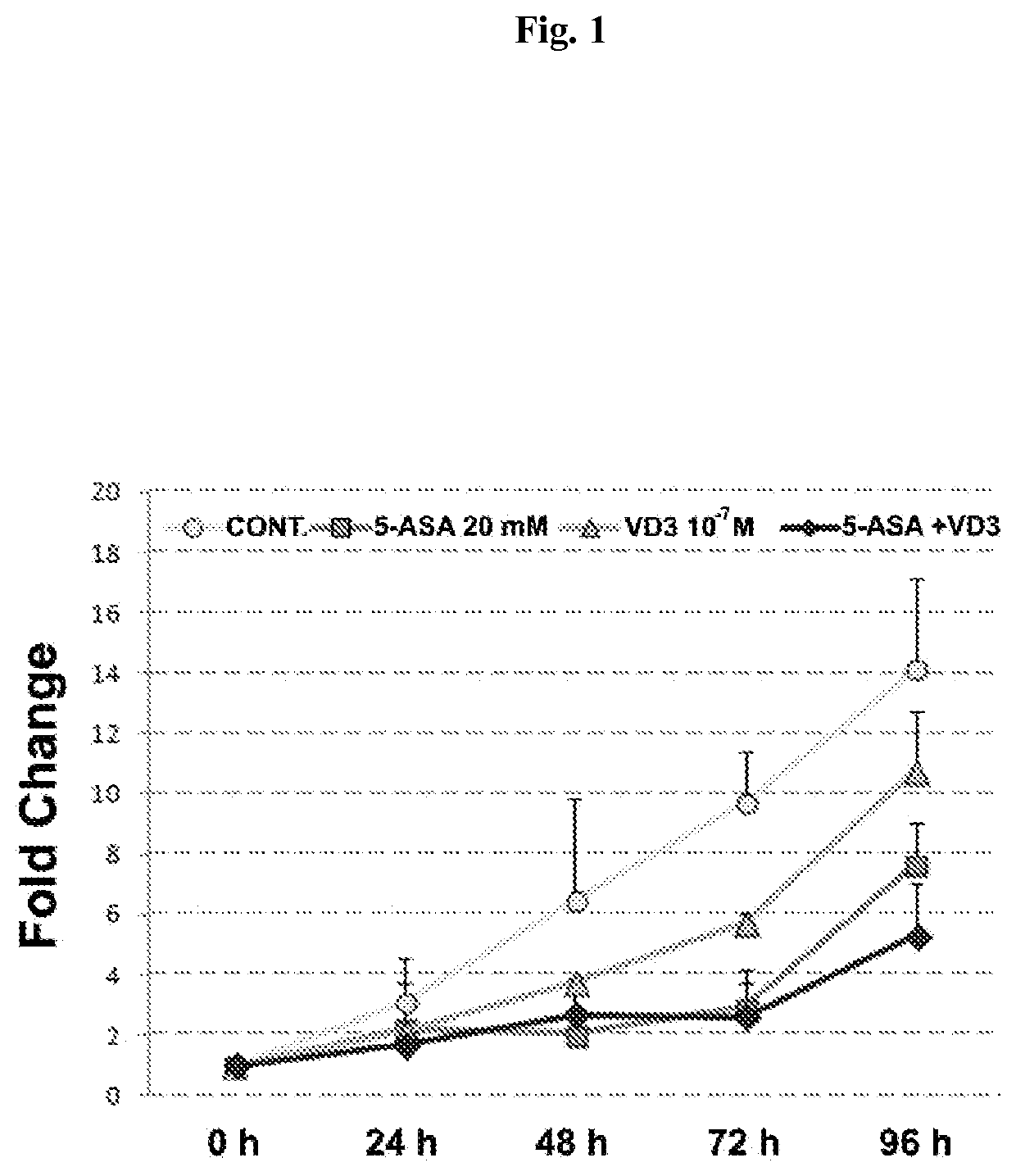
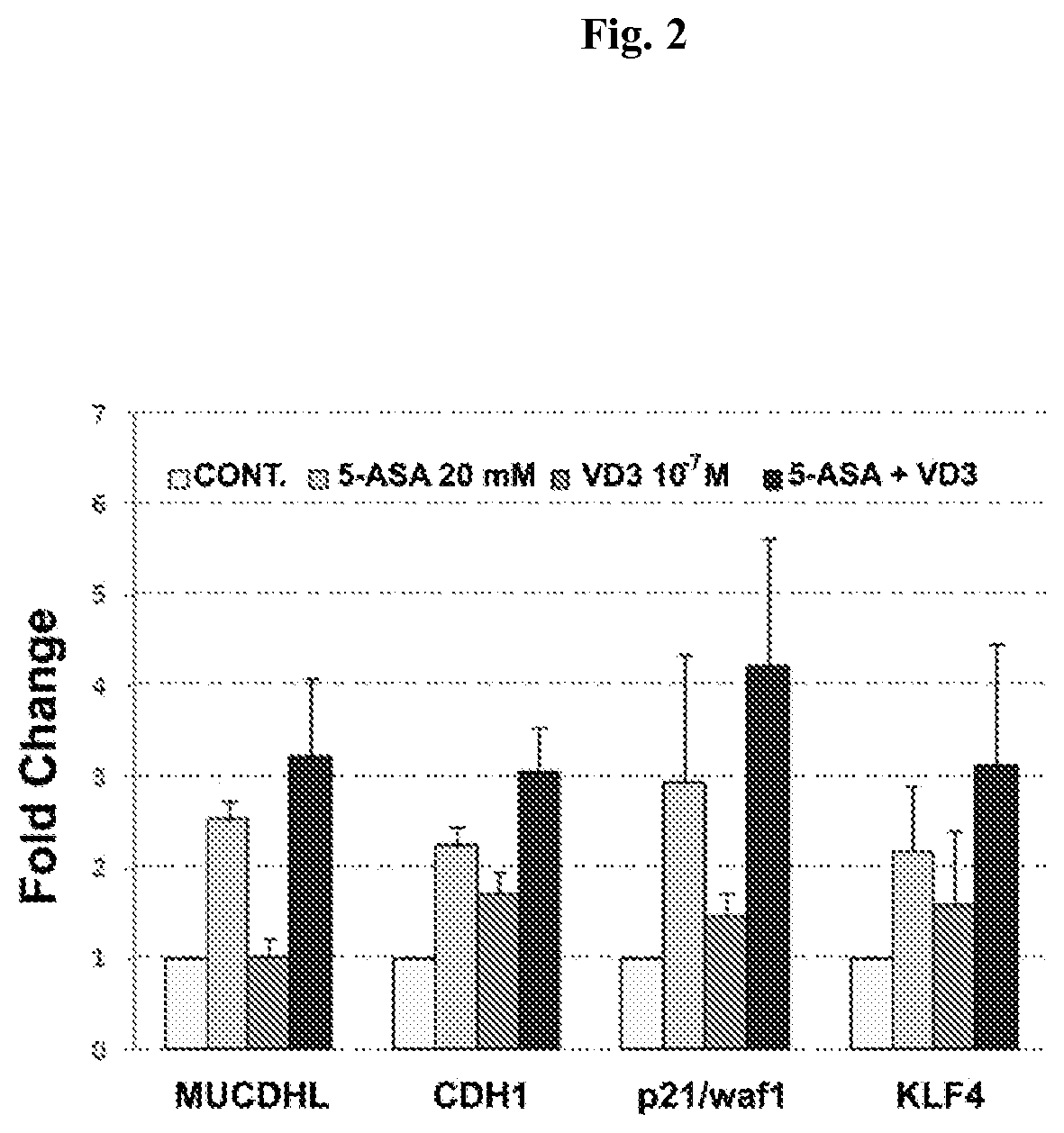
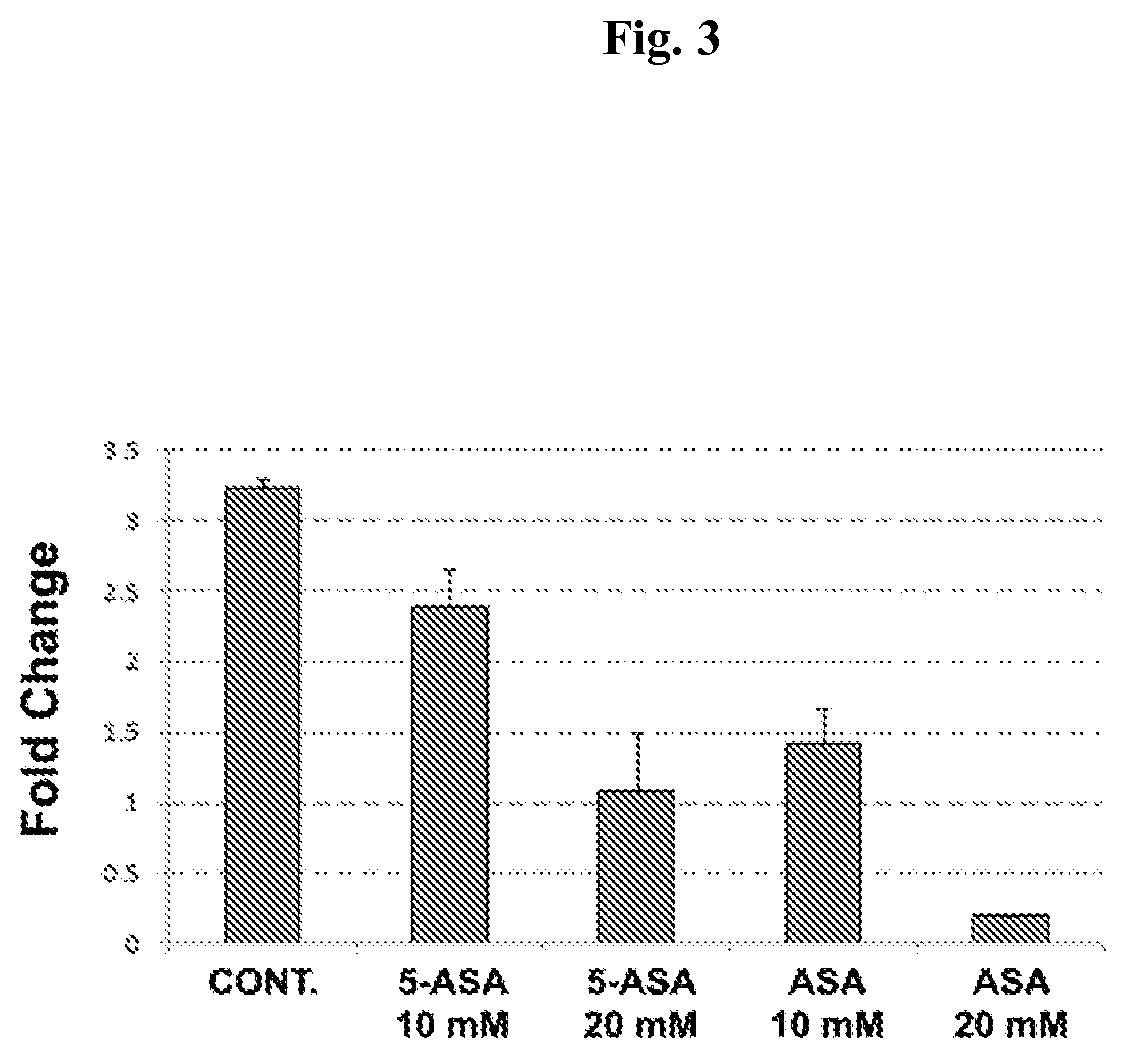
Chemoprevention of colorectal cancer
ActiveUS20150164921A1Effect be exertLow therapeutic doseOrganic active ingredientsBiocideD vitaminsExcipient
In the present invention, a new combination is disclosed comprising (i) 5-aminosalicylic acid (5-ASA) or a derivative thereof, or a pharmacologically acceptable salt thereof, and (ii) a group D vitamin, a derivative thereof, a metabolite or analogue, for use in the prevention and / or treatment of colorectal cancer (CRC). A further aspect of the invention is directed to pharmaceutical compositions comprising said combination together with at least one physiologically acceptable excipient and the use thereof in the prevention and / or in the treatment of the colorectal cancer.
Owner:ALFASIGMA SPA
Chemoprevention of colorectal cancer
ActiveUS10555956B2Low therapeutic doseOrganic active ingredientsDigestive systemMetaboliteColon rectal cancer
In the present invention, a new combination is disclosed comprising (i) 5-aminosalicylic acid (5-ASA) or a derivative thereof, or a pharmacologically acceptable salt thereof, and (ii) a group D vitamin, a derivative thereof, a metabolite or analogue, for use in the prevention and / or treatment of colorectal cancer (CRC). A further aspect of the invention is directed to pharmaceutical compositions comprising said combination together with at least one physiologically acceptable excipient and the use thereof in the prevention and / or in the treatment of the colorectal cancer.
Owner:ALFASIGMA SPA
Lymph purification therapeutic apparatus
InactiveCN105497999AAvoid damageReduce disturbanceMedical devicesSuction devicesTherapy relatedThrombus
The invention discloses a lymph purification therapeutic apparatus provided with a thoracic duct lymph drainage tube. An end of the thoracic duct lymph drainage tube includes three branches that are connected with a drainage tube pressure sensitive cuff, a drive pump and a lymph purifier outlet; the driving pump is connected with a suction jar that is connected with a lymph purifier inlet; the lymph purifier is connected with a displacement liquid feeder. This apparatus drains all lymph out from the intestinal system, thus avoiding blood entry; low disturbance is caused to the internal environment; the anticoagulation problem is weakened, and the risk of bleeding and clotting is greatly reduced; a therapeutic dose is low; a treatment period is shortened; the influence of bedside blood purification therapy on drug removal can be reduced, and high efficient drug concentration in blood is maintained, thus improving therapeutic effect of drugs such as antibiotics, reducing dosage of the antibiotics, reducing drug resistance and lowering drug therapy related costs.
Owner:张伟
Application of traditional Chinese medicine composition to preparation of drug for treating allergic conjunctivitis
InactiveCN104491013AGood curative effectImprove efficiencySenses disorderPill deliveryToxic materialBiology
The invention discloses application of a traditional Chinese medicine composition to preparation of a drug for treating allergic conjunctivitis, relating to the technical field of traditional Chinese medicines. The traditional Chinese medicine composition is prepared from Chinese herbal medicines such as honeysuckle, semen cassiae, semen plantaginis, scutellaria baicalensis, mint, equisetum hyemale, climbing groundsel herbs and liquorice. The traditional Chinese medicine composition disclosed by the invention is high in effect taking speed, short in treatment course and high in cure rate and can be popularized and applied on the aspect of treating allergic conjunctivitis. The traditional Chinese medicine composition disclosed by the invention can take a synergistic effect and simultaneously take the effects of dispelling wind and evils, clearing away heat and toxic materials as well as dispelling wind and arresting itching.
Owner:GUANGDONG JUZHICHENG TECH
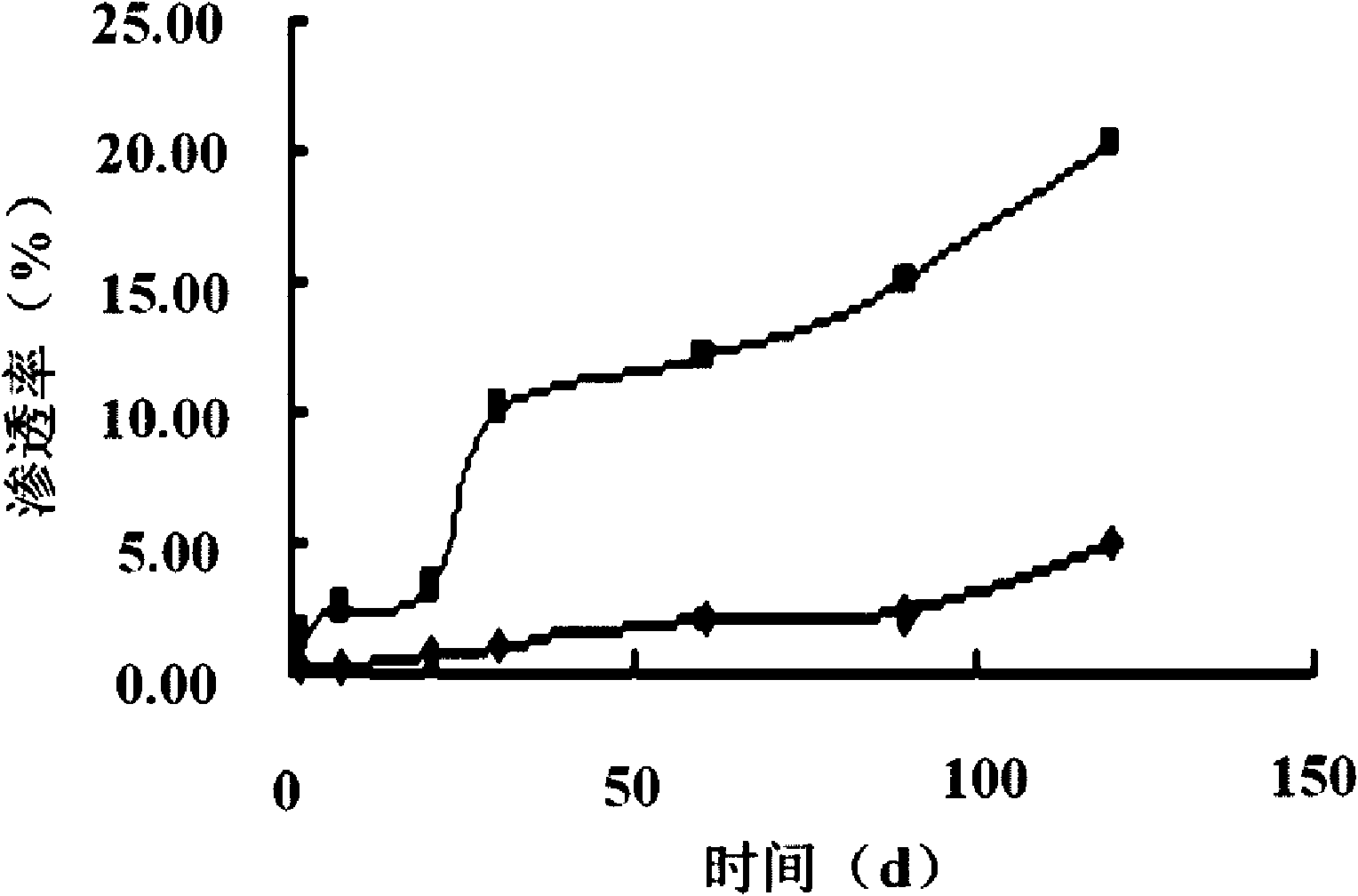
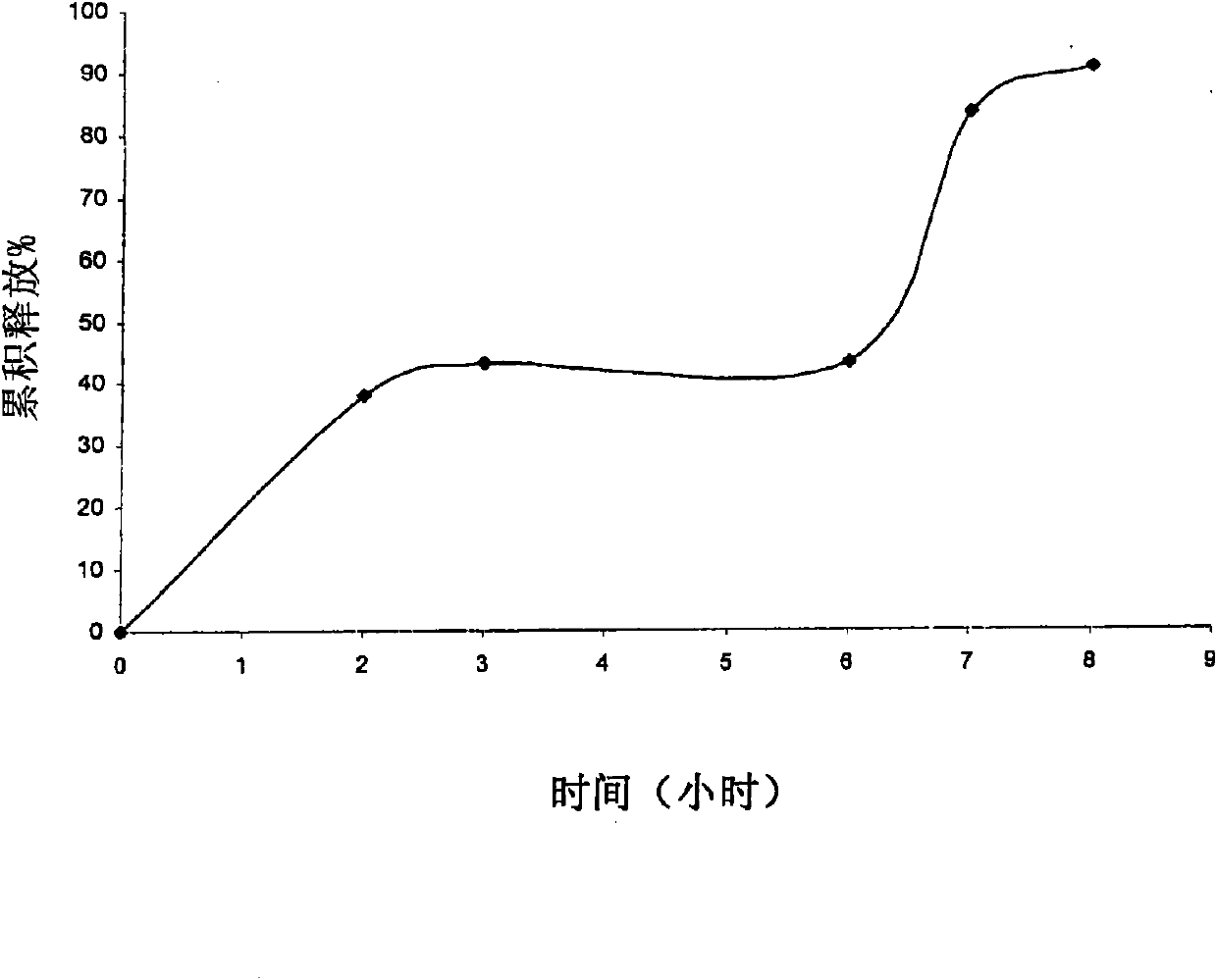
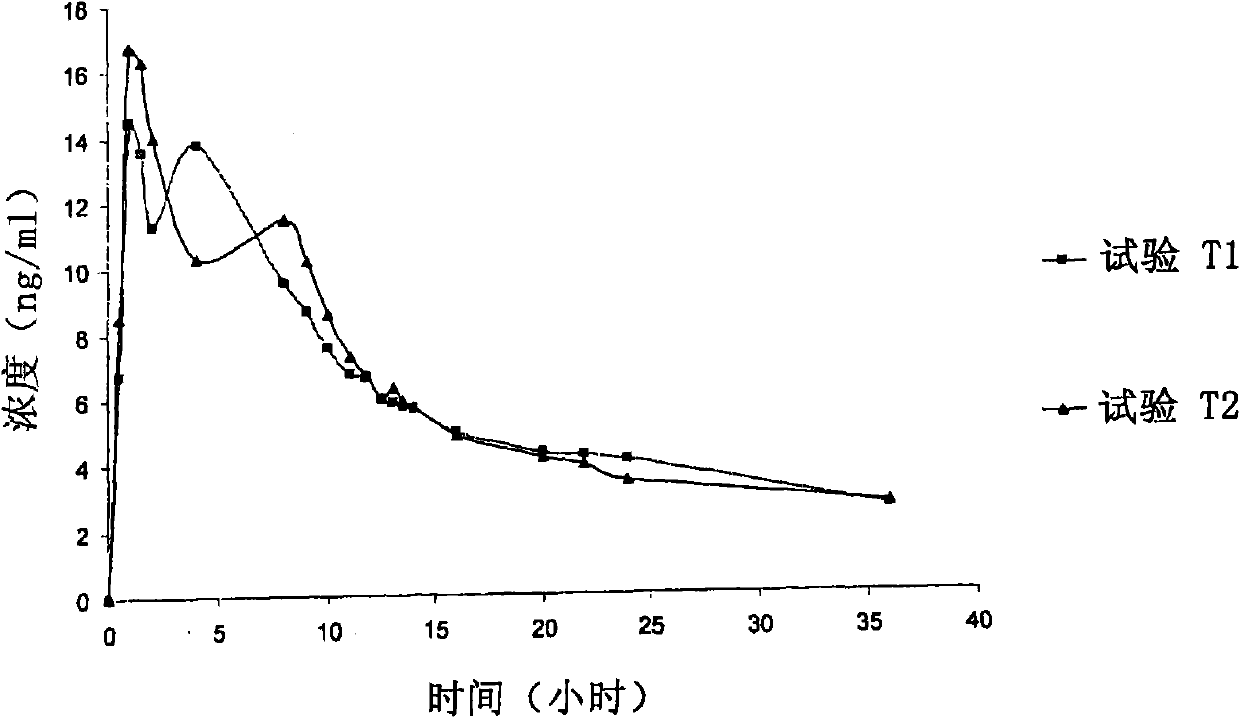
Application of 3,5-dinitrophenyl-pyrazolo[3,4-d][1,3]oxazine as a tumor drug resistance reversal agent
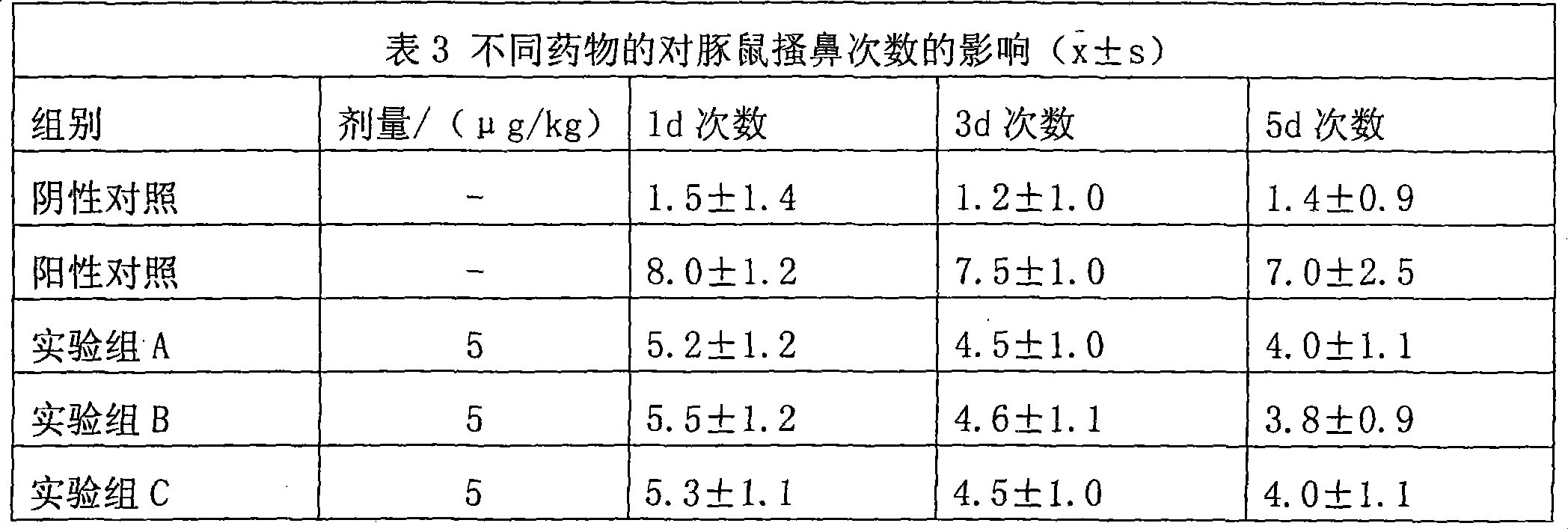
ActiveCN112402425BLow therapeutic doseEasy to useOrganic active ingredientsAntineoplastic agentsChemotherapy combinationsOncology
The invention belongs to the technical field of medicine and relates to the application of 3,5-dinitrophenyl-pyrazolo[3,4-d][1,3]oxazine as a tumor drug resistance reversal agent. Research of the present invention shows that 1-phenyl-6-(3,5-dinitrophenyl)-pyrazolo[3,4-d][1,3]oxazin-4(1H)-ketone can significantly improve The sensitivity of tumor cells to anti-tumor drugs can reverse the drug resistance of tumor cells, and the treatment dose is safe and non-toxic, which has the potential to become a new type of tumor drug resistance reversal agent. Based on the results of this study, the above-mentioned compounds can be used as adjuvant therapy of chemotherapy regimens and co-administered with other anti-tumor drugs, which has good clinical significance.
Owner:无锡享源信息科技有限公司
Application of 3, 5-dinitrophenyl-pyrazolo [3, 4-d] [1,3] oxazine as tumor drug resistance reversal agent
ActiveCN112402425ALow therapeutic doseEasy to useOrganic active ingredientsAntineoplastic agentsOxazinesKetone
The invention belongs to the technical field of medicines, and relates to an application of 3, 5-dinitrophenyl-pyrazolo [3, 4-d] [1,3] oxazine as a tumor drug resistance reversal agent. Researches show that 1-phenyl-6-(3, 5-dinitrophenyl)-pyrazolo[3, 4-d] [1, 3] oxazine-4(1H)-ketone can significantly improve the sensitivity of tumor cells to antitumor drugs and reverse the drug resistance of the tumor cells, and the treatment dosage is safe and non-toxic, so that the compound has potential to become a novel tumor drug resistance reversal agent. Based on the research results, the compound can be used for adjuvant therapy of a chemotherapy regimen and can be matched with other anti-tumor drugs for common medication, and has good clinical medication significance.
Owner:无锡享源信息科技有限公司
Niobium tungstate compound for resisting hepatitis virus
InactiveCN101844811BSynthetic raw materials are readily availableLow priceHeavy metal active ingredientsOrganic chemistryTungstateNiobium
The invention provides a niobium tungstate compound for resisting hepatitis virus, which has the function of resisting the hepatitis virus and the chemical formula of AmBr (X (NbO2) 3W9O37). nH2O, wherein A is alkali metal ion Li, Na, K or Cs; B is hydrogen cation H+, ammonium ion NH4+ or amino acid cation; X is Si, Ge or P; and m=0-7, r=0-7 and n=0-20. The niobium tungstate compound is taken as active ingredient which is assisted by carrier or auxiliary material that is pharmaceutically acceptable, thus being used for preparing oral preparation or injection medicine applied to treating hepatitis. Compared with the existing medicine which is applied to treating hepatitis clinically, the niobium tungstate compound has the advantages of better liver target, low effective therapeutic dose, higher therapeutic index and lower toxicity, thus being an antiviral compound which has higher efficiency and is safer.
Owner:JILIN UNIV
Hydroxyl camptothecin flexible liposome and preparation method thereof
InactiveCN102397254BGood curative effectLow therapeutic doseOrganic active ingredientsPharmaceutical non-active ingredientsWater bathsCholesterol
The invention discloses a hydroxyl camptothecin flexible liposome and a preparation method thereof. The method comprises the following steps of: dissolving hydroxyl camptothecin and carrier materials, such as phospholipid, cholesterol and the like, in an organic solvent; forming the mixture into a membrane in water bath through rotary evaporation; and adding buffer solution which contains chitosan into the membrane to form the hydroxyl camptothecin flexible liposome. The hydroxyl camptothecin flexible liposome is relatively lower in toxicity, relatively longer in residence time in a body, high in stability in a solution phase and smalllow in particle size, and the particle size of the liposome is not obviously changed after the liposome is lyophilized.
Owner:GUANGXI ZHUANGDU BIO TECH
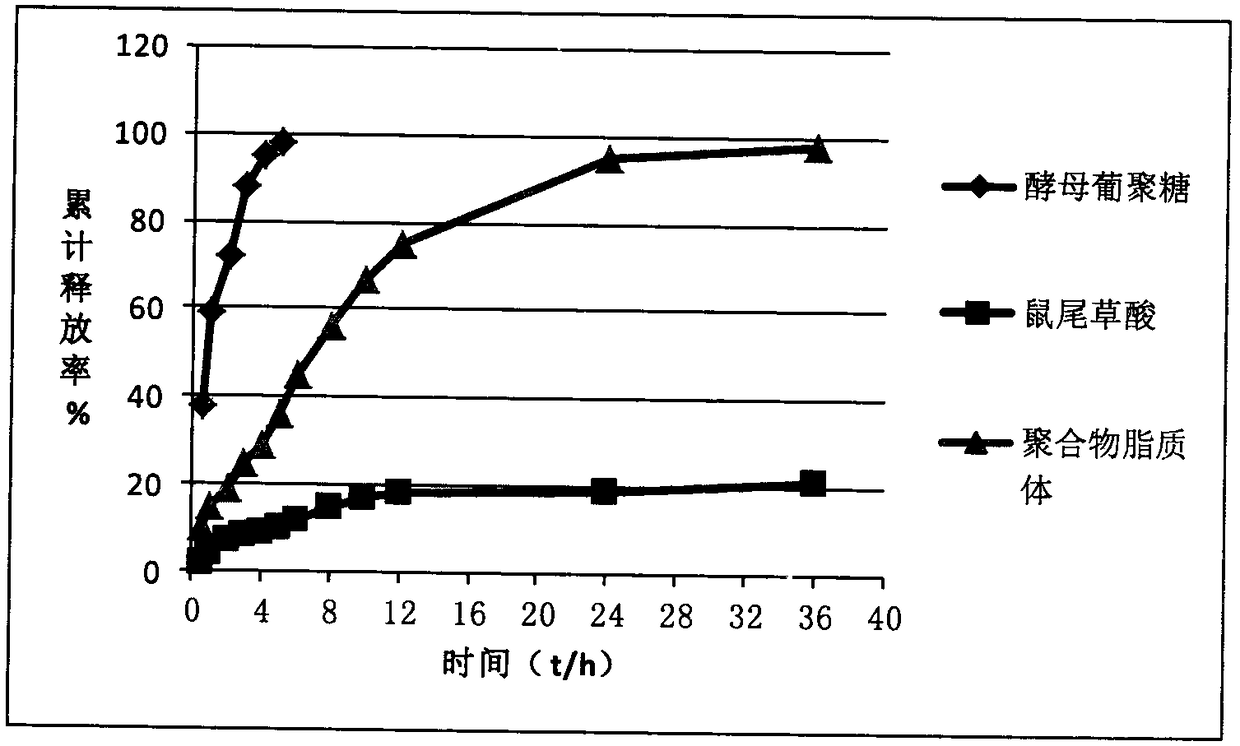
Polymer thermosensitive liposome loaded with yeast glucan and carnosic acid
InactiveCN108904450AReduce penetrationImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsThermosensitive liposomesCancer cell
The invention relates to a polymer thermosensitive liposome loaded with yeast glucan and carnosic acid and a preparation method thereof. The polymer thermosensitive liposome comprises the following raw materials by weight: 5-15 parts of the yeast glucan, 5-10 parts of the carnosic acid, 4-10 parts of polylysine, 25-80 parts of phospholipid, 2-5 parts of tocopherol and 50-160 parts of cholesterol.The two drugs are respectively encapsulated in different positions of a lipid bilayer by membrane dispersion technology, and prescription technology and physicochemical properties are optimized and investigated to prepare a nanostructured carrier with excellent properties, and the polymer thermosensitive liposome can reverse multidrug resistance of cancer cells.
Owner:GUANGZHOU JIAYUAN PHARMA TECH
A kind of pharmaceutical composition containing inorganic arsenic compound and its application
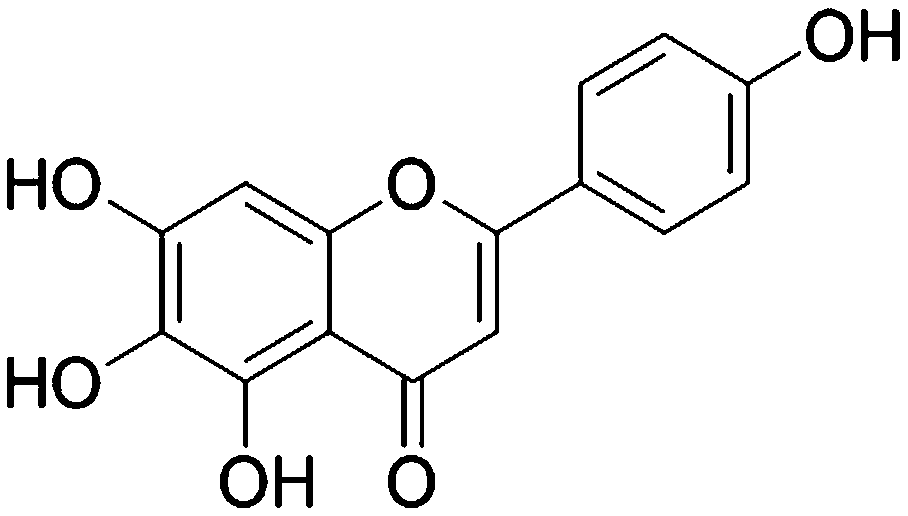
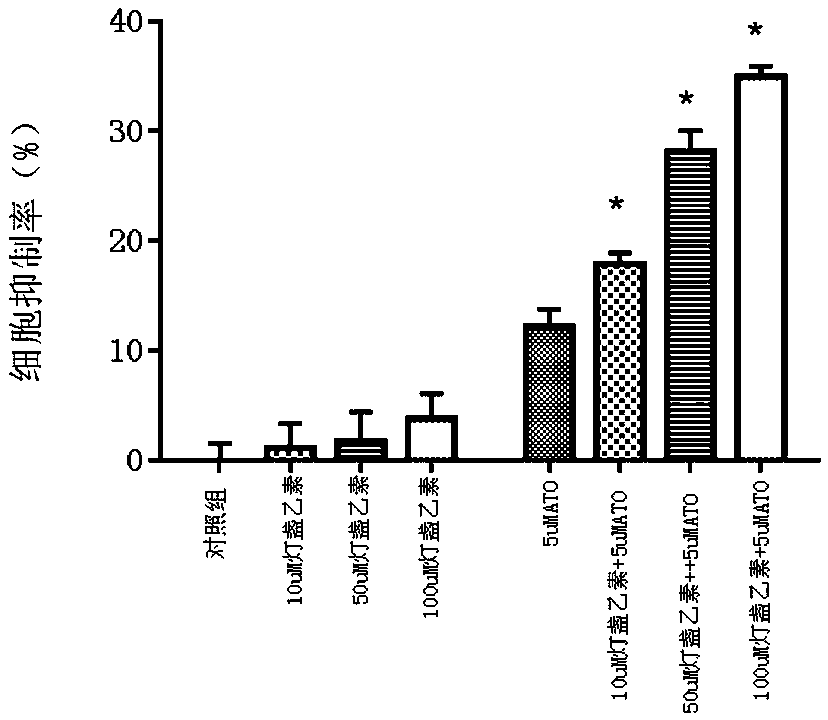
ActiveCN106214698BEnhanced inhibitory effectLow therapeutic doseOrganic active ingredientsInorganic active ingredientsInorganic arsenicCancer cell
The invention discloses a medicine composition containing inorganic arsenic compounds and application thereof. The medicine composition comprises inorganic arsenic compounds, scutellarin or scutellarein, is used for preparing medicines for treating cancers, and has a better effect on cancer cell inhibition than that by singly using inorganic arsenic compounds and that by singly using scutellarin or scutellarein. According to the medicine composition, the treatment dosage of inorganic arsenic compounds can be reduced, the cancer inhibition effect can be enhanced, and the systematic toxin of inorganic arsenic compounds can be effectively reduced.
Owner:NANTONG YUFUYUAN PHARMA CO LTD
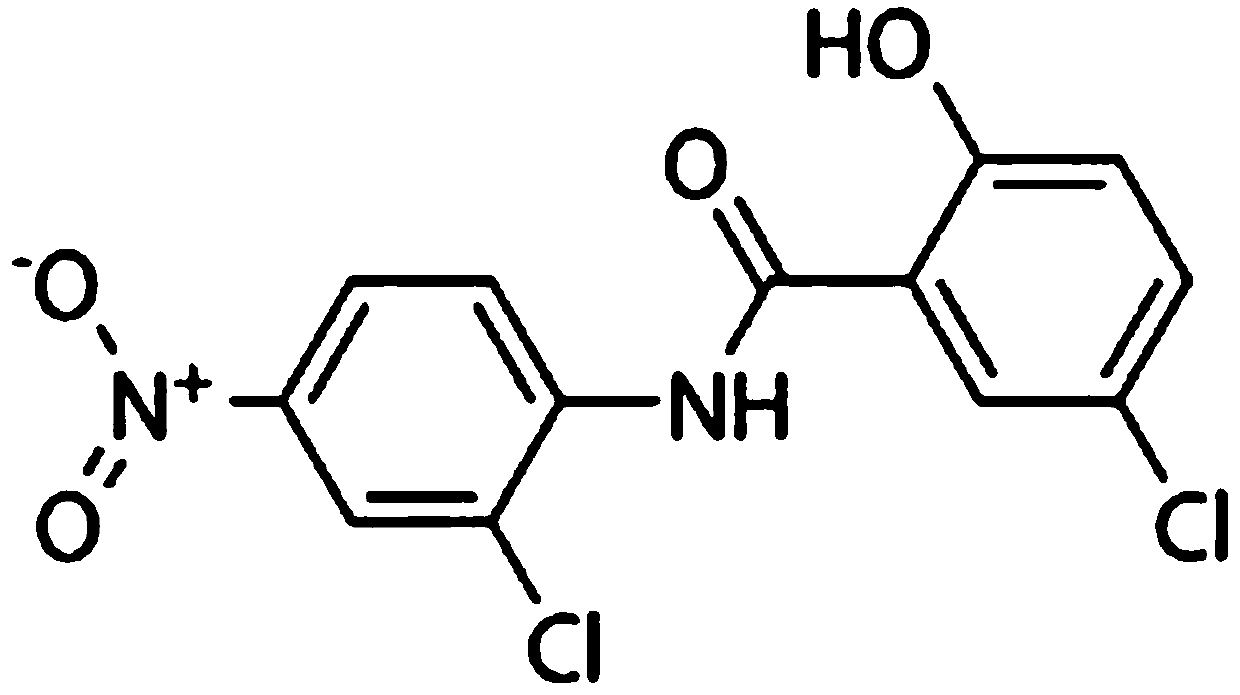
A kind of pharmaceutical composition containing inorganic arsenic compound and its application
ActiveCN107184600BEnhanced inhibitory effectLow therapeutic doseOrganic active ingredientsInorganic active ingredientsInorganic arsenicCancer cell
The invention discloses a pharmaceutical composition containing an inorganic arsenic compound and application of the pharmaceutical composition. The pharmaceutical composition contains the inorganic arsenic compound and Niclosamide and is used for preparing a cancer treatment medicine. Niclosamide has the molecular formula of C13H8Cl2N2O4 and the CAS number of 50-65-7. The pharmaceutical composition has a stronger cancer cell killing function than that of independent use of the inorganic arsenic compound or Niclosamide. The therapeutic dose of the inorganic arsenic compound is reduced, the cancer inhibition effect is enhanced, and the systematic poisonousness of the inorganic arsenic compound is effectively reduced.
Owner:中芝堂药业(山东)有限公司
Protein vaccine against tumor necrosis factor alpha and use thereof
ActiveCN106540252BInhibit biological functionLow therapeutic doseAntipyreticAntibody mimetics/scaffoldsAutoimmune conditionMutated protein
The invention belongs to the field of biological medicines, and relates to a mutant protein vaccine aiming at tumor necrosis factor alpha (TNF-alpha) and applications of the mutant protein vaccine. The invention aims at providing the protein vaccine which is good in property and takes TNF-alpha as the target spot. According to the scheme, by immunizing the TNF-alpha mutant protein vaccine, the biological activity of the TNF-alpha mutant protein vaccine is inhibited, the cross immunologic reaction is generated in vivo, the biological functions of TNF-alpha are neutralized, and the autoimmune diseases and inflammatory diseases are inhibited. The protein vaccine aiming at TNF-alpha can be used for treating the autoimmune diseases and the inflammatory diseases and preventing the relapse of the autoimmune diseases and the inflammatory diseases.
Owner:SICHUAN UNIV
Nano-drug carrier, nano-drug preparation, preparation method and application
PendingCN112220929AHigh drug loadingHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryBio moleculesDrug administration
The invention relates to a nano-drug carrier, which comprises a polyethyleneimine derivative, wherein the fatty acid modification degree of the polyethyleneimine derivative is 0.1-12%, and the biological molecular weight range of the polyethyleneimine derivative is 0-25kDa. The invention also discloses a nano-drug preparation prepared from the nano-drug carrier, a preparation method and application. The nano-drug carrier provided by the invention has the advantages of high drug loading capacity, high encapsulation efficiency, good biocompatibility and the like, is safe and nontoxic, is free from immunogenicity and is biodegradable, a drug treatment dosage is greatly reduced, in addition, the nano-drug carrier can slowly release drugs on a targeting position so as to perform a curative effect for a long time, drug administration frequencies are greatly reduced, and therefore, a toxic and side effect of the drug is further lowered.
Owner:HUBEI UNIV
RNA encoding protein
PendingCN113454227AEffective secretionEfficient deliveryPolypeptide with localisation/targeting motifMuscular disorderDiseaseNucleic acid sequencing
The present invention relates to a mRNA comprising a nucleic acid sequence encoding a protein and a signal peptide and a transcription unit, an expression vector or a gene therapy vector comprising a nucleic acid encoding the protein and a signal peptide. Also disclosed herein is a therapeutic composition comprising the mRNA, transcription unit, expression vector or gene therapy vector and use of the therapeutic composition in treating a disease or a condition.
Owner:维萨梅布有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![Application of 2-phenylpyrazole [1,5-a] pyrimidine compounds serving as tumor drug resistance reversal agent Application of 2-phenylpyrazole [1,5-a] pyrimidine compounds serving as tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bd264cf8-3451-44a8-a86c-7f5b2264eeac/HDA0002465515070000011.png)
![Application of 2-phenylpyrazole [1,5-a] pyrimidine compounds serving as tumor drug resistance reversal agent Application of 2-phenylpyrazole [1,5-a] pyrimidine compounds serving as tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bd264cf8-3451-44a8-a86c-7f5b2264eeac/FDA0002465515050000011.png)
![Application of 2-phenylpyrazole [1,5-a] pyrimidine compounds serving as tumor drug resistance reversal agent Application of 2-phenylpyrazole [1,5-a] pyrimidine compounds serving as tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bd264cf8-3451-44a8-a86c-7f5b2264eeac/BDA0002465515060000021.png)



![Application of 3,5-dinitrophenyl-pyrazolo[3,4-d][1,3]oxazine as a tumor drug resistance reversal agent Application of 3,5-dinitrophenyl-pyrazolo[3,4-d][1,3]oxazine as a tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035ed55c-3cdb-496b-a050-726518fa87a4/BDA0002801145890000021.png)
![Application of 3,5-dinitrophenyl-pyrazolo[3,4-d][1,3]oxazine as a tumor drug resistance reversal agent Application of 3,5-dinitrophenyl-pyrazolo[3,4-d][1,3]oxazine as a tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035ed55c-3cdb-496b-a050-726518fa87a4/BDA0002801145890000041.png)
![Application of 3,5-dinitrophenyl-pyrazolo[3,4-d][1,3]oxazine as a tumor drug resistance reversal agent Application of 3,5-dinitrophenyl-pyrazolo[3,4-d][1,3]oxazine as a tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035ed55c-3cdb-496b-a050-726518fa87a4/BDA0002801145890000061.png)
![Application of 3, 5-dinitrophenyl-pyrazolo [3, 4-d] [1,3] oxazine as tumor drug resistance reversal agent Application of 3, 5-dinitrophenyl-pyrazolo [3, 4-d] [1,3] oxazine as tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0532e125-cad2-48b8-ad1e-77e801386b75/FDA0002801145880000011.png)
![Application of 3, 5-dinitrophenyl-pyrazolo [3, 4-d] [1,3] oxazine as tumor drug resistance reversal agent Application of 3, 5-dinitrophenyl-pyrazolo [3, 4-d] [1,3] oxazine as tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0532e125-cad2-48b8-ad1e-77e801386b75/FDA0002801145880000012.png)
![Application of 3, 5-dinitrophenyl-pyrazolo [3, 4-d] [1,3] oxazine as tumor drug resistance reversal agent Application of 3, 5-dinitrophenyl-pyrazolo [3, 4-d] [1,3] oxazine as tumor drug resistance reversal agent](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0532e125-cad2-48b8-ad1e-77e801386b75/FDA0002801145880000021.png)