Meloxicam tablets for dogs and cats and preparation method for meloxicam tablets
A technology for meloxicam and dogs and cats, applied in the field of medicine, can solve the problems of low absorption rate of suspensions, prone to medical accidents, and high prices of imported medicines, and achieves increased dissolution effect, improved palatability, and is convenient to take. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] This embodiment provides a meloxicam tablet for dogs and cats, especially for meloxicam tablets for dogs and cats. The meloxicam for dogs and cats in this embodiment is a tablet-type drug. It mainly consists of the following mass percentages: meloxicam: 0.1-0.6%, lactose: 50-70%, microcrystalline cellulose: 20-40%, hydroxypropyl cellulose 3-5%, sodium citrate: 0.4- 0.6%, crospovidone: 1-5%, pvpK30: 4-8%, aspartame: 0.2-0.8%, magnesium stearate: 0.60-2%.
[0095] Wherein, meloxicam is a kind of enolic acid non-steroidal anti-inflammatory drug, the inventor finds in the research process of the present invention: meloxicam can selectively inhibit cyclooxygenase-2 (COX-2 ), its inhibitory effect on COX-2 is 10-70 times stronger than that on COX-1.
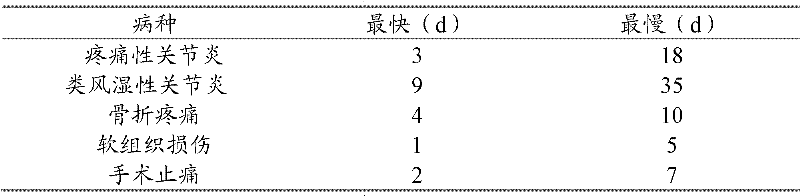
[0096] Specifically, for dogs and cats, when osteoarthritis, rheumatoid arthritis, acute or chronic fasciitis, fracture, soft tissue injury and surgery, etc., will eventually cause local congestion of the body, tissue cell necr...
Embodiment 2
[0117] A meloxicam tablet for dogs and cats, especially for dogs and cats, provided in this practice, includes main ingredients and auxiliary materials, formulated according to the following weight percentages: main ingredient 0.29%, auxiliary materials 99.7%. The meloxicam tablet of the present invention is formulated by the raw material of following mass percentage:
[0118] Meloxicam: 3 grams;
[0119] Lactose: 600 grams;
[0120] Microcrystalline cellulose: 284 grams;
[0121] Hydroxypropyl cellulose: 40 grams;
[0122] Sodium citrate: 5 grams;
[0123] Cross-linked PVP: 30 grams;
[0124] PVPK30: 60 grams;
[0125] Aspartame: 5 grams;
[0126] Magnesium stearate: 10 grams;
[0127] A total of 1500 tablets were produced.
[0128] The preparation method of the meloxicam tablet of the present invention comprises the weighing and mixing of raw materials and the tableting process, and is characterized in that the specific process steps are as follows:
[0129] 1) Put la...
Embodiment 3
[0136] The present embodiment provides a meloxicam tablet for dogs and cats, especially for dogs and cats, including main ingredients and auxiliary materials, formulated according to the following weight percentages: main ingredient 0.1%, auxiliary materials 99.9%.
[0137] The meloxicam tablet of the present invention is formulated by the raw material of following mass percentage:
[0138] Meloxicam: 1 g
[0139] Lactose: 579 grams
[0140] Microcrystalline cellulose: 272 grams
[0141] Hypromellose: 35 grams
[0142] Sodium citrate: 5 grams
[0143] Cross-linked PVP: 33 grams
[0144] PVPK30: 58 grams
[0145] Aspartame: 4 grams
[0146] Magnesium stearate: 13 grams
[0147] A total of 1500 tablets were produced.
[0148] The preparation method of the meloxicam tablet of the present invention comprises the weighing and mixing of raw materials and the tableting process, and is characterized in that the specific process steps are as follows:
[0149] 1) Put lactose, m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com