Methods and compositions for optimizing blood and tissue stability of camptothecin and other albumin-binding therapeutic compounds
a technology of albumin-binding compounds and albumin-binding compounds, which is applied in the direction of drug compositions, heterocyclic compound active ingredients, biocide, etc., can solve the problems of affecting clinical utility, pharmacophore significantly compromising clinical utility, and affecting clinical utility, so as to enhance the free drug levels of camptothecin and its free drug levels. , the effect of enhancing the free drug level of the therapeutic compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
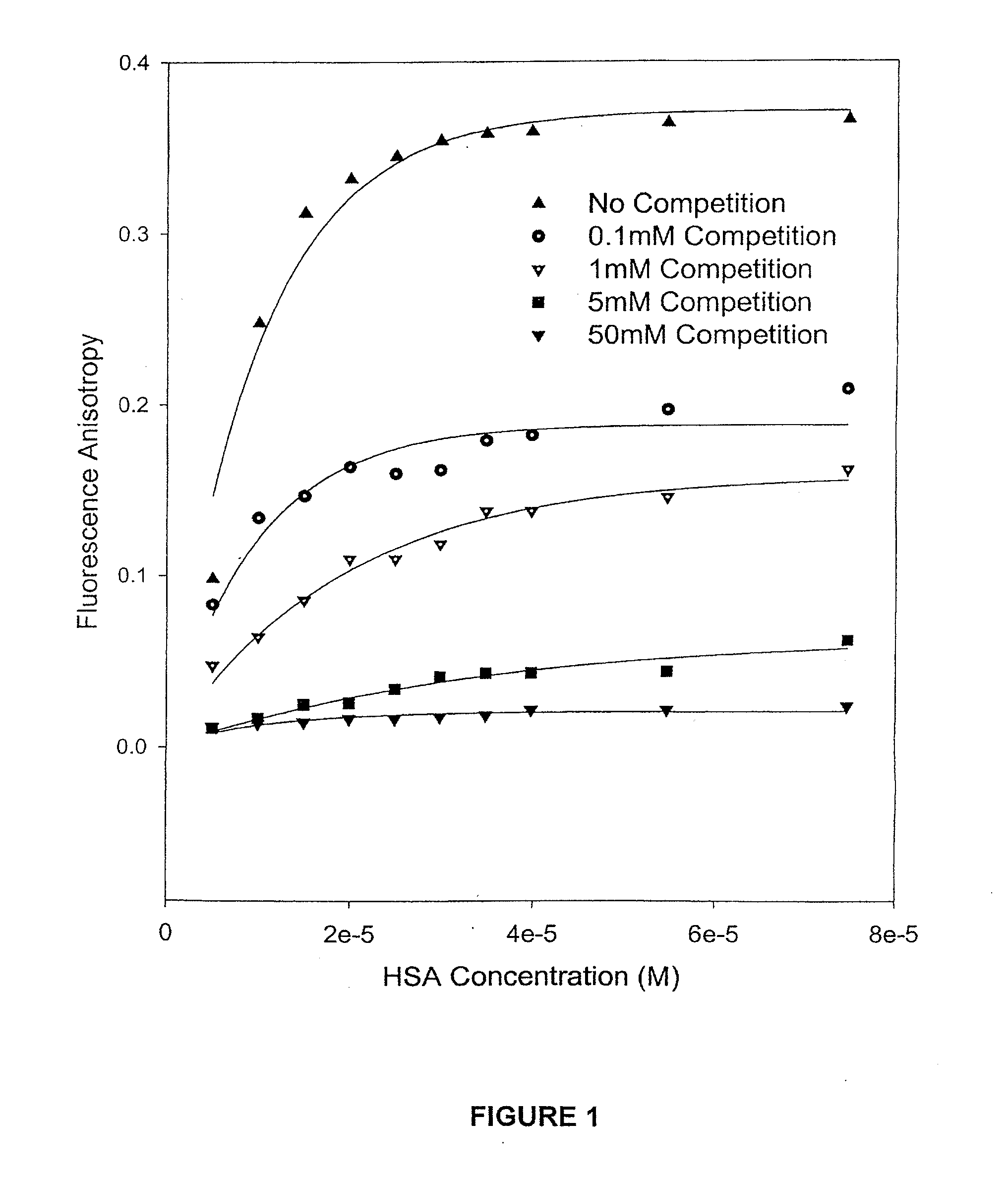
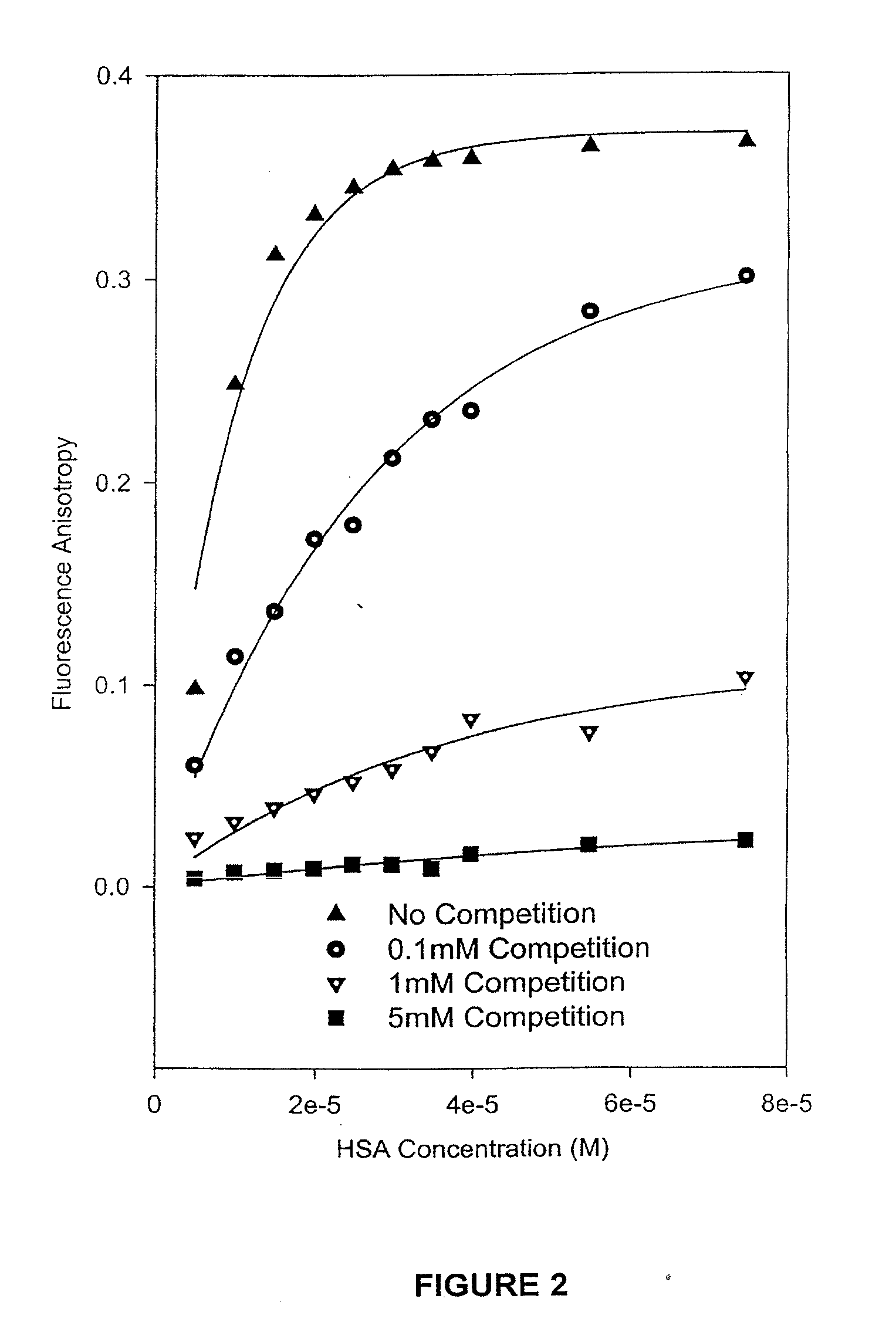
HSA / Competition Experiments by Fluorescence Spectroscopic Methods
Materials and Methods:
[0063]The camptothecin used in the experiments was obtained from Boehringer Ingelhem (Lot#95-002). Dimethyl Sulfoxide (HPLC grade, Aldrich, Milwaukee, Wis.) was used to prepare stock solutions of camptothecin at various concentrations, which were stored in the dark at −20° C. Working solutions of 1.0×10−3 M camptothecin carboxylate and camptothecin lactone were prepared by diluting a stock solution of camptothecin in DMSO with PBS buffer at pH values of 10.0 and 3.0, respectively. The Sigma Chemical Co. (St. Louis, Mo.) supplied the human serum albumin (HSA) employed in the binding experiments. A 2.5×10−3 M stock solution of HSA was prepared in PBS buffer at a final pH of 7.40±0.05. The concentration of the HSA was determined on a weight-to-volume basis (g / L). A Milli-Q UV PLUS purification system (Bedford, Mass.) was used to acquire high-purity water.
[0064]For the competition binding experiments,...
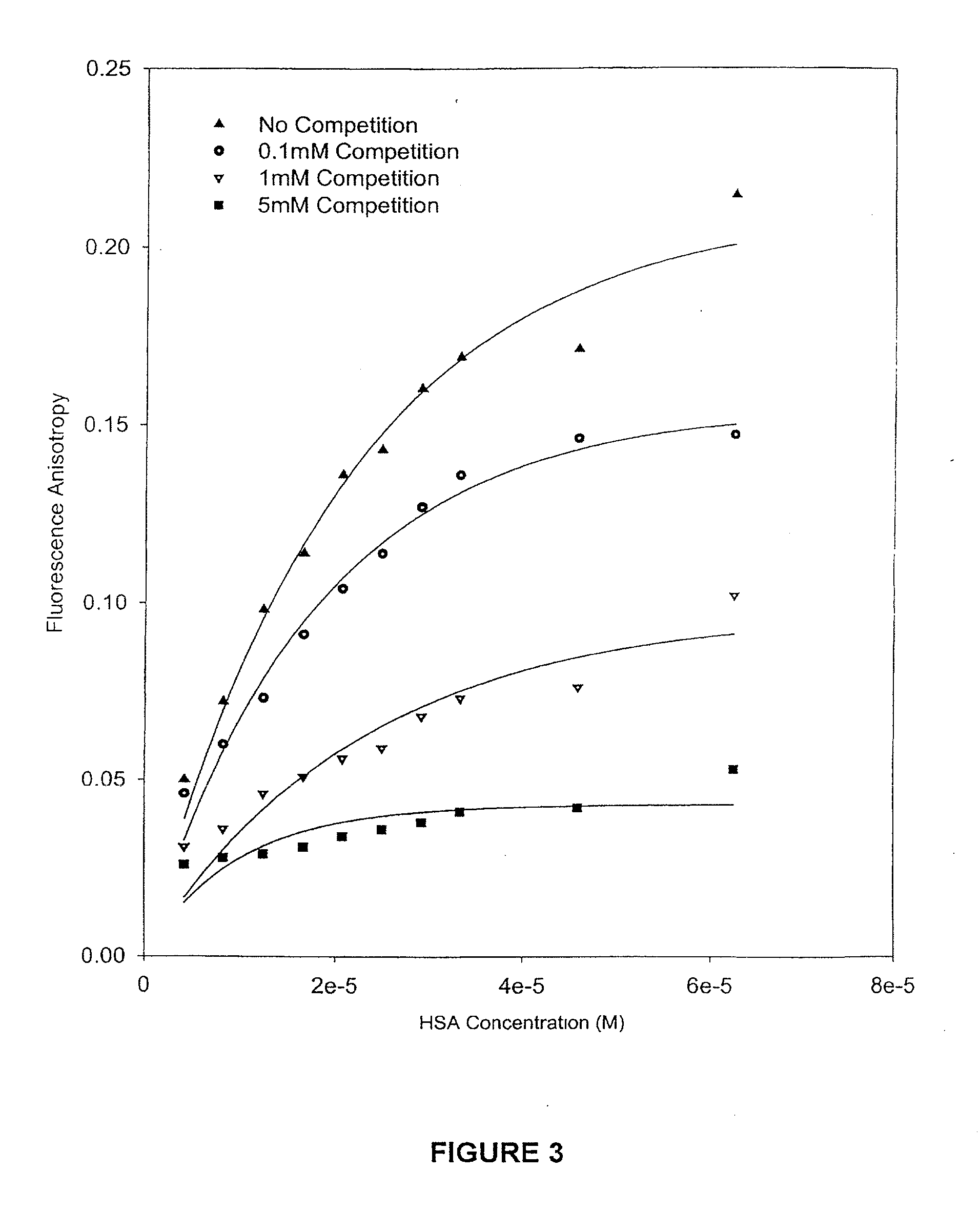
example 2
Procedure of Competition Binding and Stability of 9AC, DB172, DB67 and SN38 with the Presence of Various Drugs
1. Materials
[0070]Samples of 9AC, DB67, DB172 and SN38 were obtained from various sources. Human serum albumin (HSA) was purchased from Sigma Chemical (St. Louis, Mo.). Recovered human plasma was obtained from Central Kentucky Blood Center (Lexington, Ky.) and stored at −20° C. Whole human blood was obtained from a healthy male donor by drawing blood into sterile vacutainers containing heparin, to prevent clot formation. Ultrafiltration tubes were purchased from Millipore. (Centrifree; MW cutoff 30,000). Triethylamine and HPLC-grade acetonitrile was purchased from Fisher Scientific (Fair Lawn, N.J., USA). High purity water was provided by a Milli-Q UV Plus purification system (Millipore, Bedford, Mass., USA). Stock solutions of each drug were prepared in A.C.S. spectrophotometric grade dimethylsulfoxide (DMSO; Aldrich, Milwaukee, Wis., USA) at a concentration of 2×10−3M and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com