Multiple sustained release vascular embolization drug-loading composition
A technology of vascular embolism and composition, which is applied in the field of multiple slow-release vascular embolism drug-loaded compositions, which can solve problems such as adverse reactions, affecting long-term therapeutic effect, and increased drug concentration, and achieve the effect of drug burst release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A drug-loaded composition for multiple sustained-release vascular embolism, the drug is released from it in 1-360 days, and the degradation time in vivo is 1-360 days, which includes drug-loaded microspheres and is made of degradable and / or non-degradable materials A three-dimensional porous scaffold, the drug-loaded microspheres are loaded in the three-dimensional porous scaffold through hydrogel or autologous blood clot, so that the drug in the drug-loaded microspheres is released from the drug-loaded microspheres to the hydrogel or autologous blood clot, and then It is then released into the three-dimensional porous scaffold, and finally released outside the three-dimensional porous scaffold. The pores of the three-dimensional porous scaffold are evenly distributed, and the pore diameter of the pores is larger than the diameter of the drug-loaded microspheres, wherein the diameter of the drug-loaded microspheres is 5nm-900μm, and the pore diameter of the pores of the ...
Embodiment 2
[0050] (1) Preparation of degradable drug-loaded microspheres (taking chitosan and gelatin microspheres as examples): after dissolving the gelatin solution / chitosan solution with a concentration of 10% on a water bath at 50-60° C. Add doxorubicin hydrochloride and mix thoroughly in a vortex mixer to make an aqueous phase. Add an appropriate amount of Span-80 to liquid paraffin to form the oil phase, place it in a three-necked bottle in a constant temperature water bath at 50°C, and stir at a speed of 200-1000r·min -1 Under certain conditions, slowly emulsify the water phase into the oil phase drop by drop, the ratio of water to oil is 1:4-1:8. Microscopic examination until the emulsion drops into a spherical shape of suitable size, that is, after emulsification to form a stable W / O emulsion, quickly cool down to below 5°C, add formaldehyde or 50% glutaraldehyde to cure for 1-2 hours, and the obtained product will be heated at 3000r / min Centrifuge, wash with isopropanol and ac...
Embodiment 3
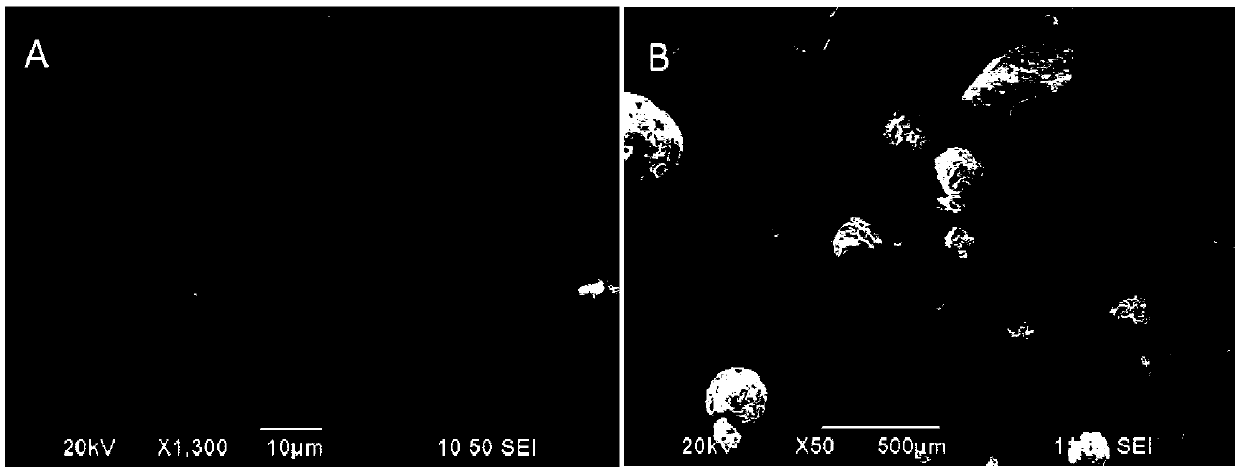
[0054](1) Preparation of non-degradable drug-loaded microspheres (taking PVA microspheres as an example): under stirring conditions, add 2 g of surfactant Span-80 to 40 mL of liquid paraffin to form a continuous oil phase; , add 10mL of PVA to the oil phase, after mixing well, add 1g of STMP as a cross-linking agent, and immediately add 1mL of NaOH as a catalyst. Set the rotation speed to 400r / min, the temperature to 50°C, and the reaction time to 16h. After the cross-linking reaction is over, let it stand for 30 minutes. Add a small amount of absolute ethanol, put it in a centrifuge for centrifugation, take out the supernatant, wash the precipitate repeatedly with absolute ethanol, isopropanol and pure water, and finally put it in a blast drying oven for 24 hours to obtain White powder, its scanning electron microscope picture is as follows Figure 6 shown.
[0055] (2) Preparation of degradable three-dimensional porous scaffold (PCL porous scaffold as an example): PCL7g, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com