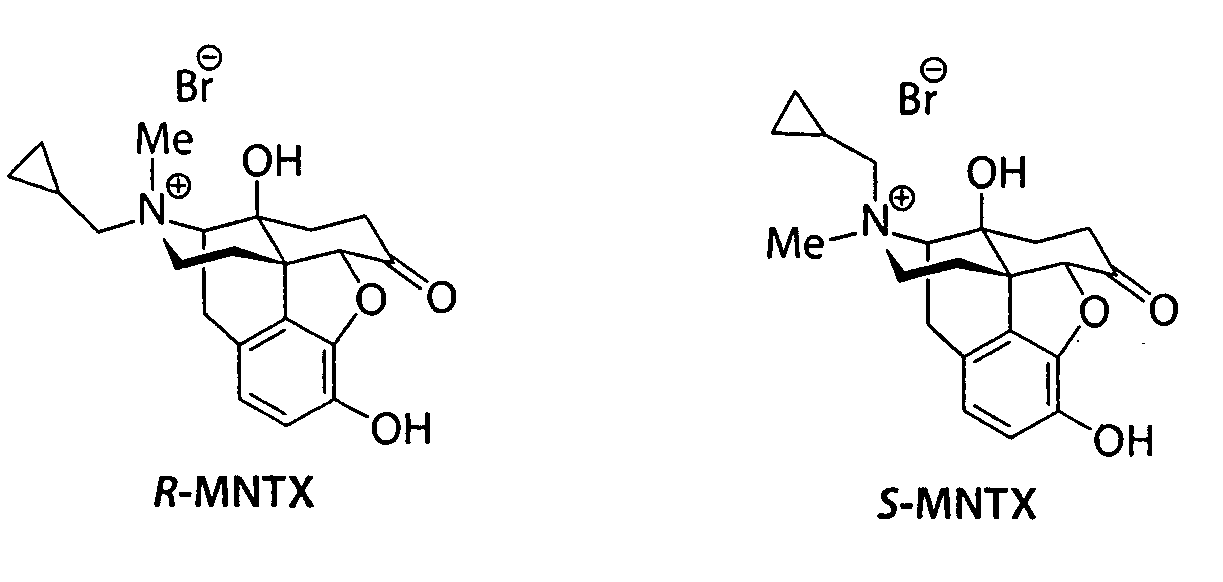
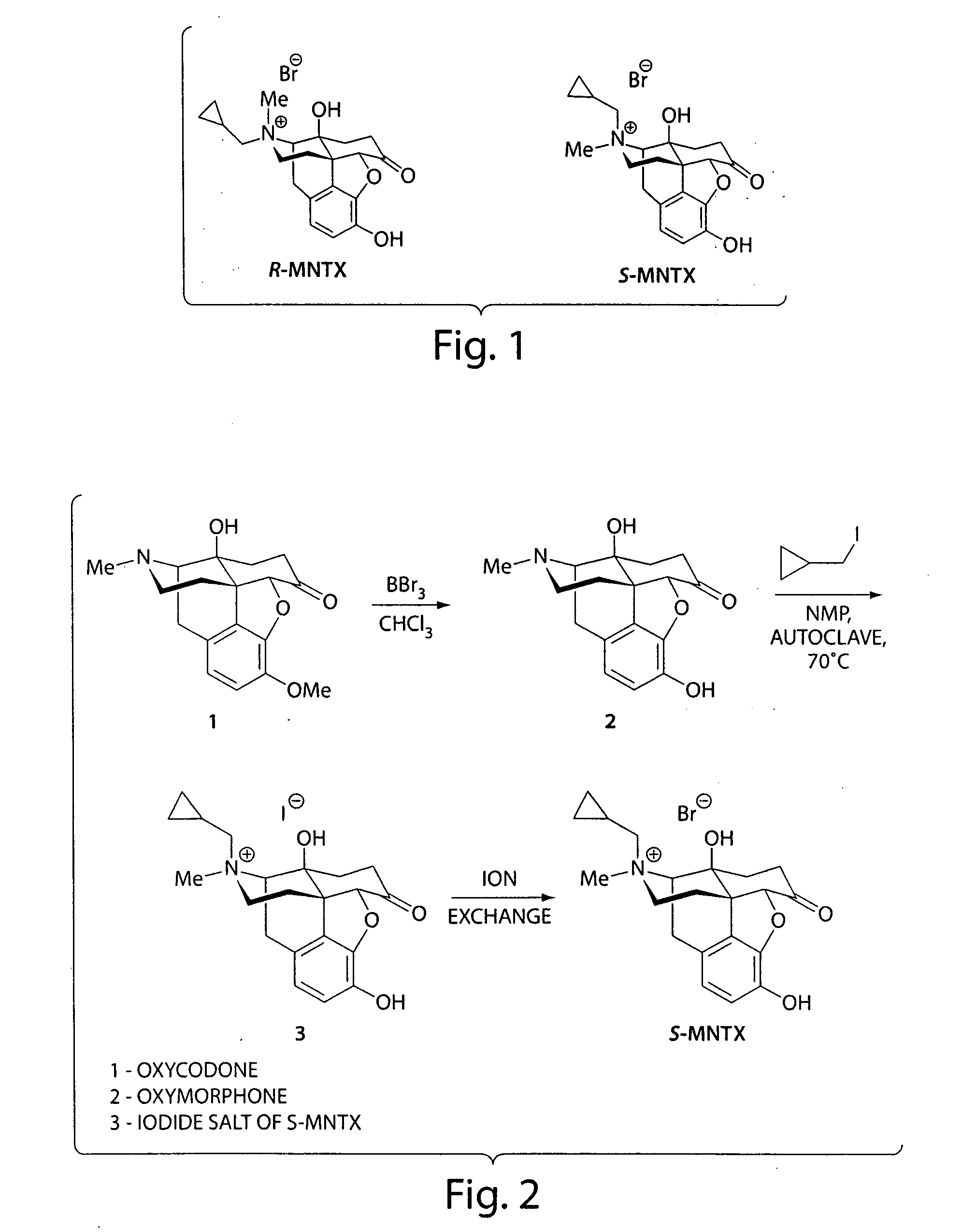
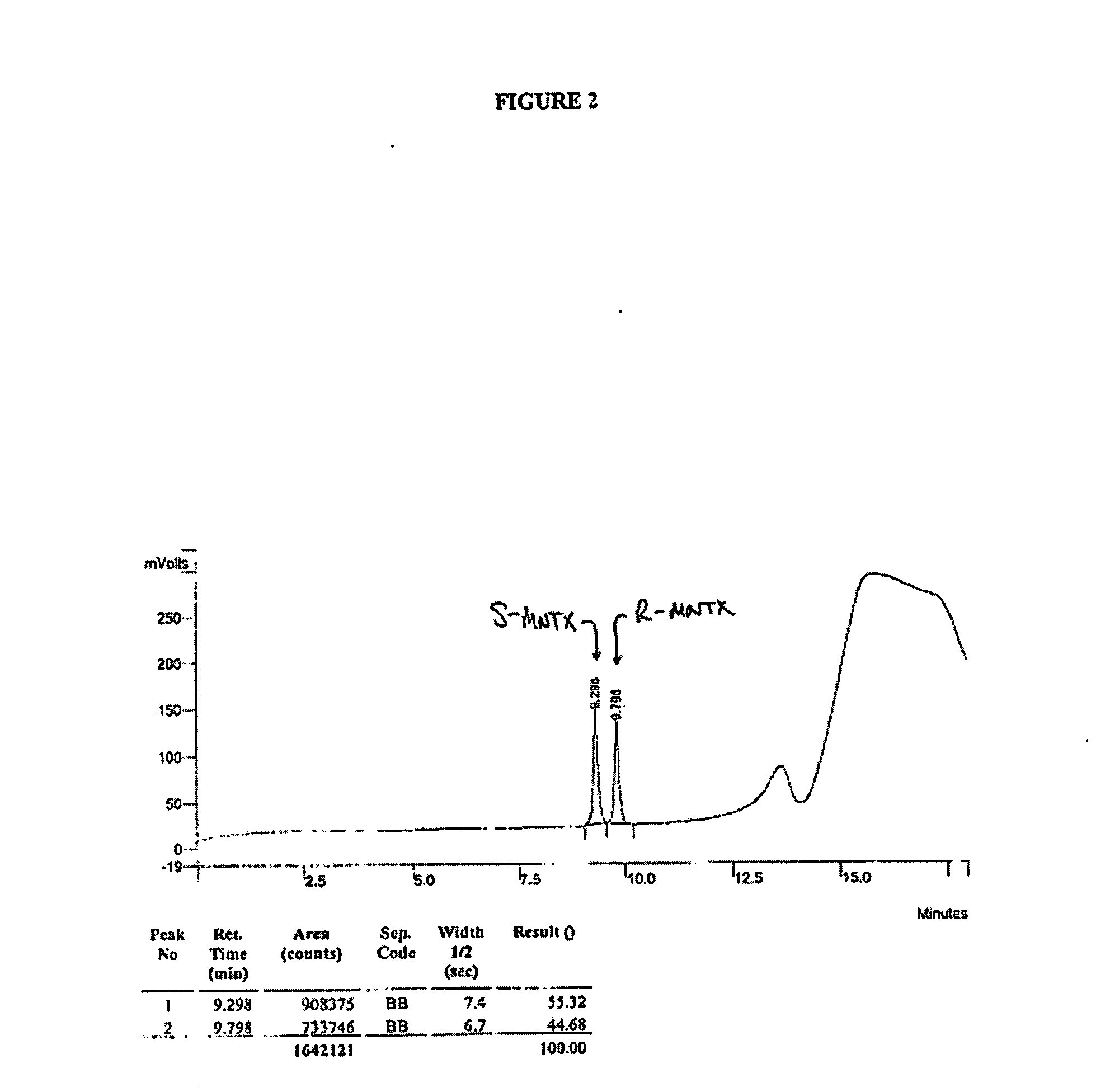
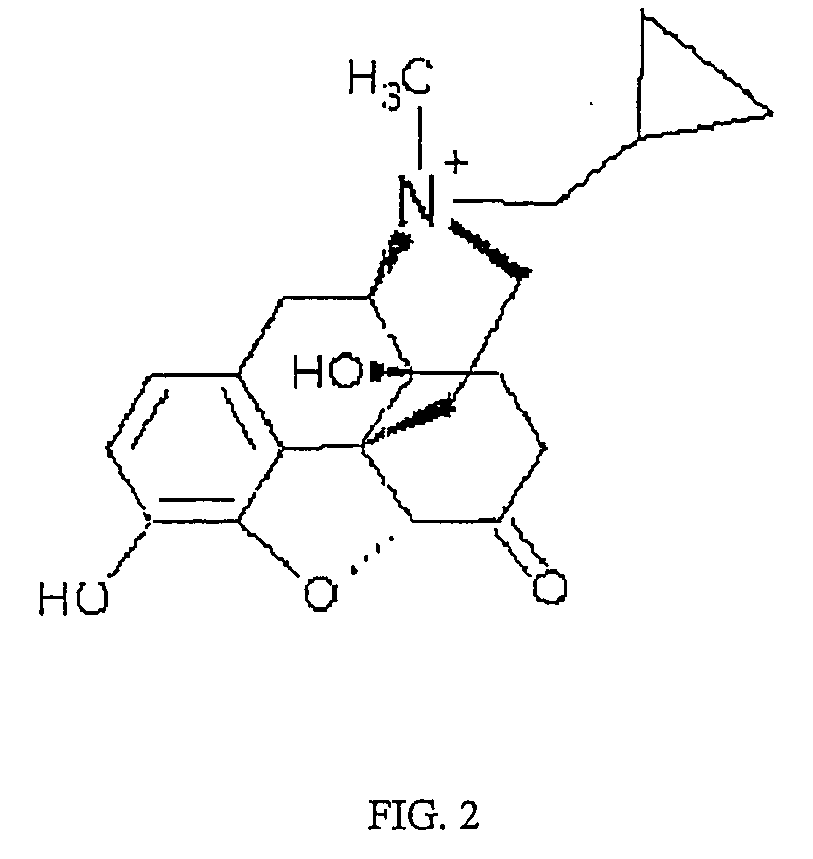
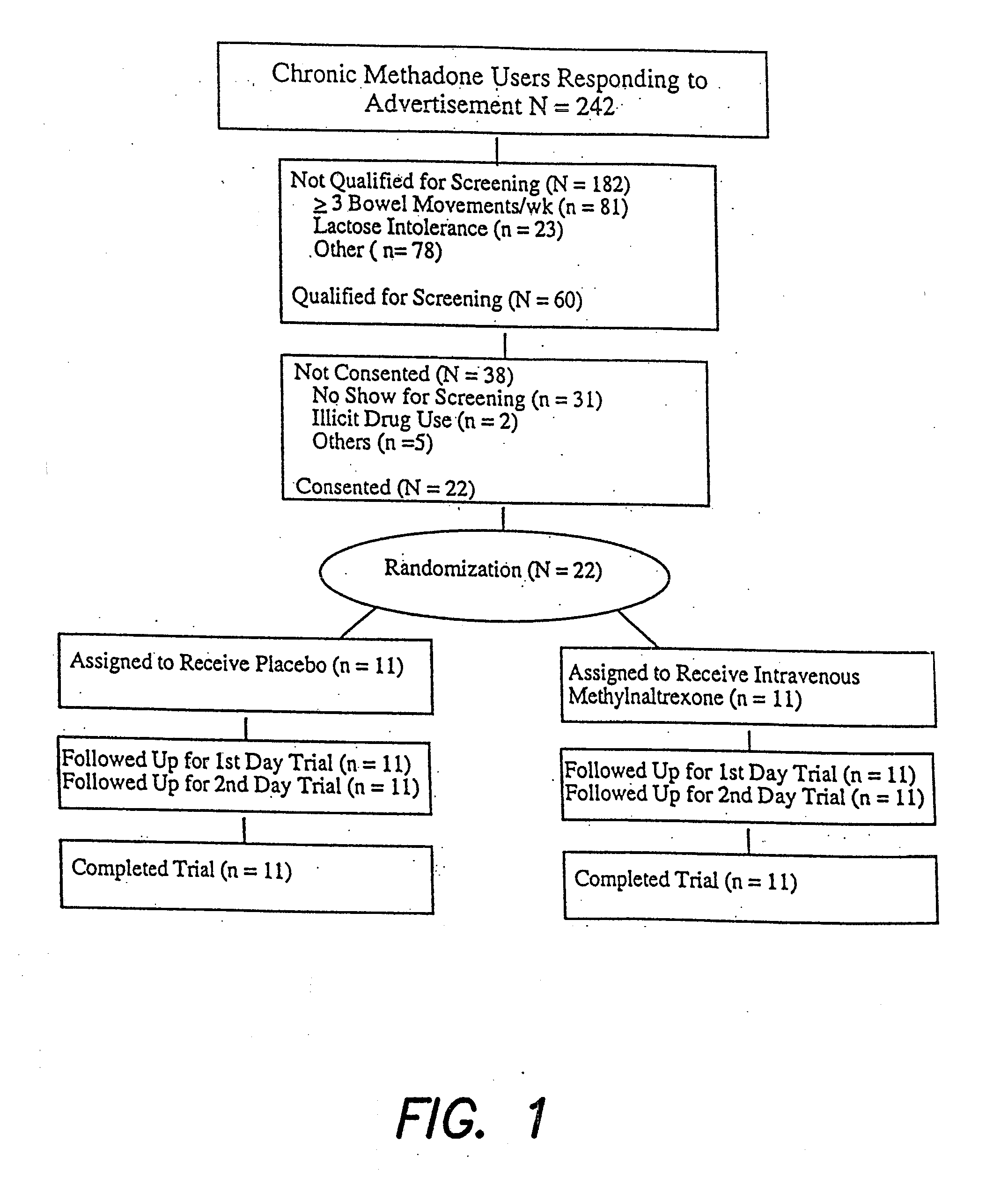
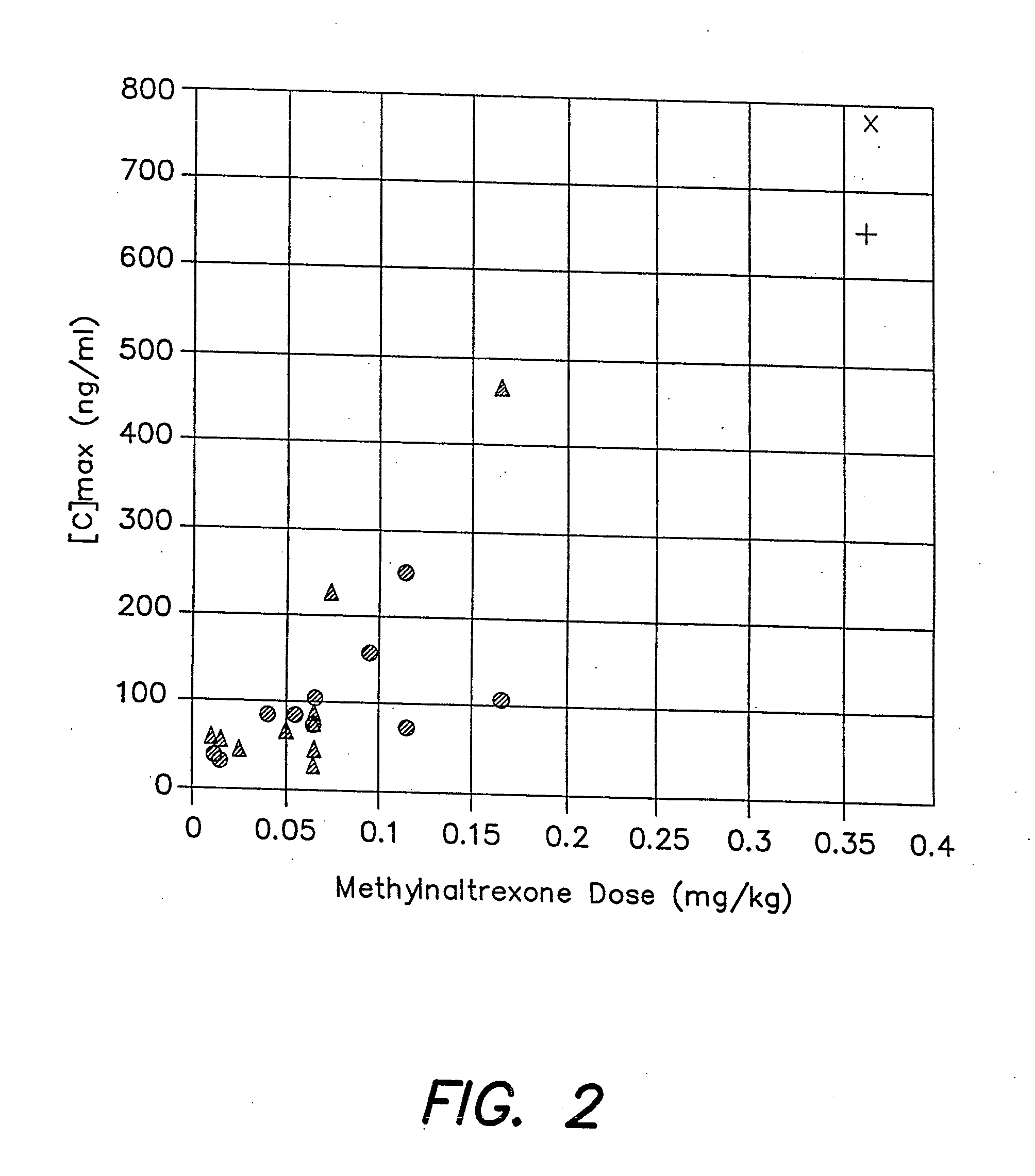
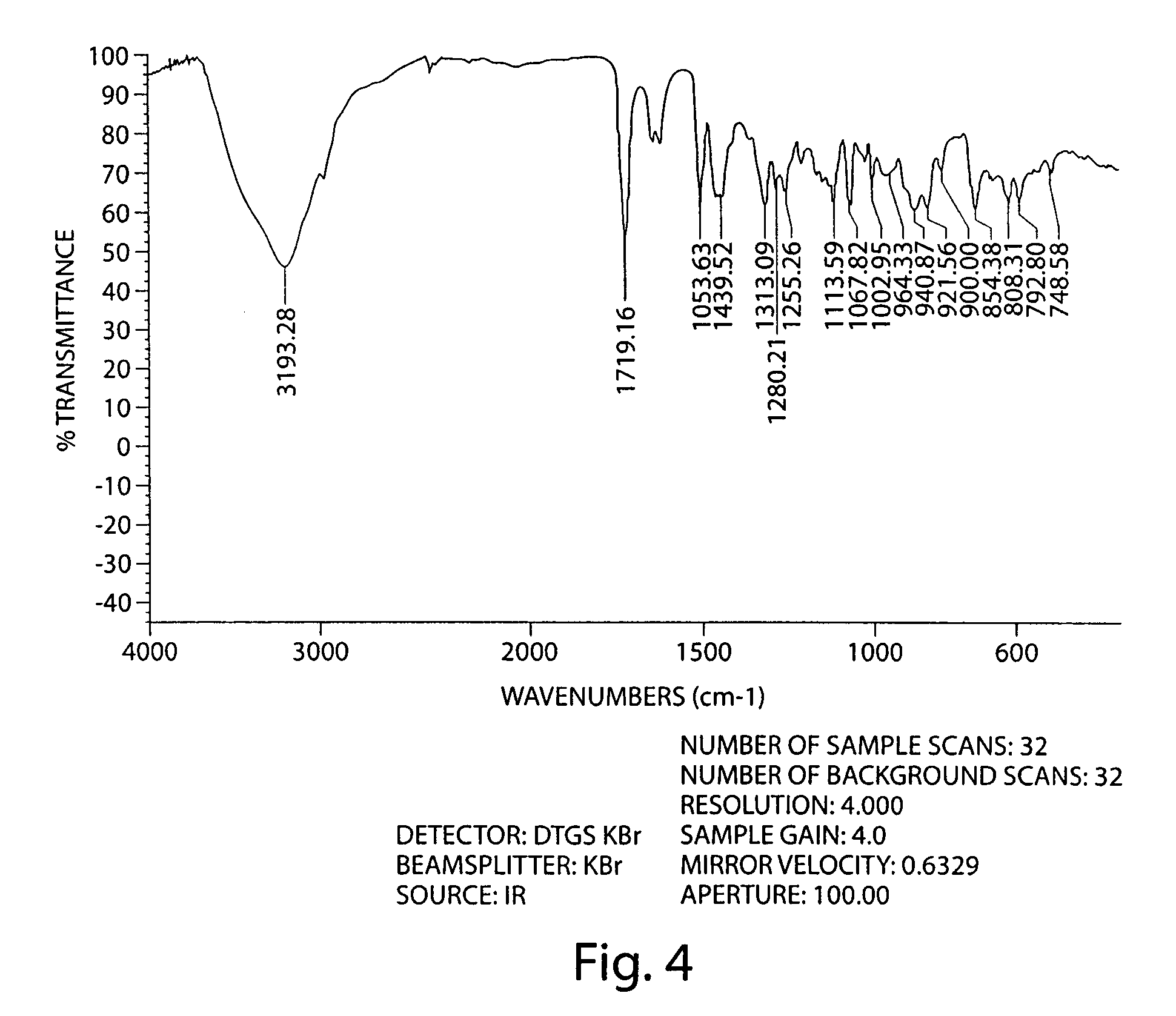
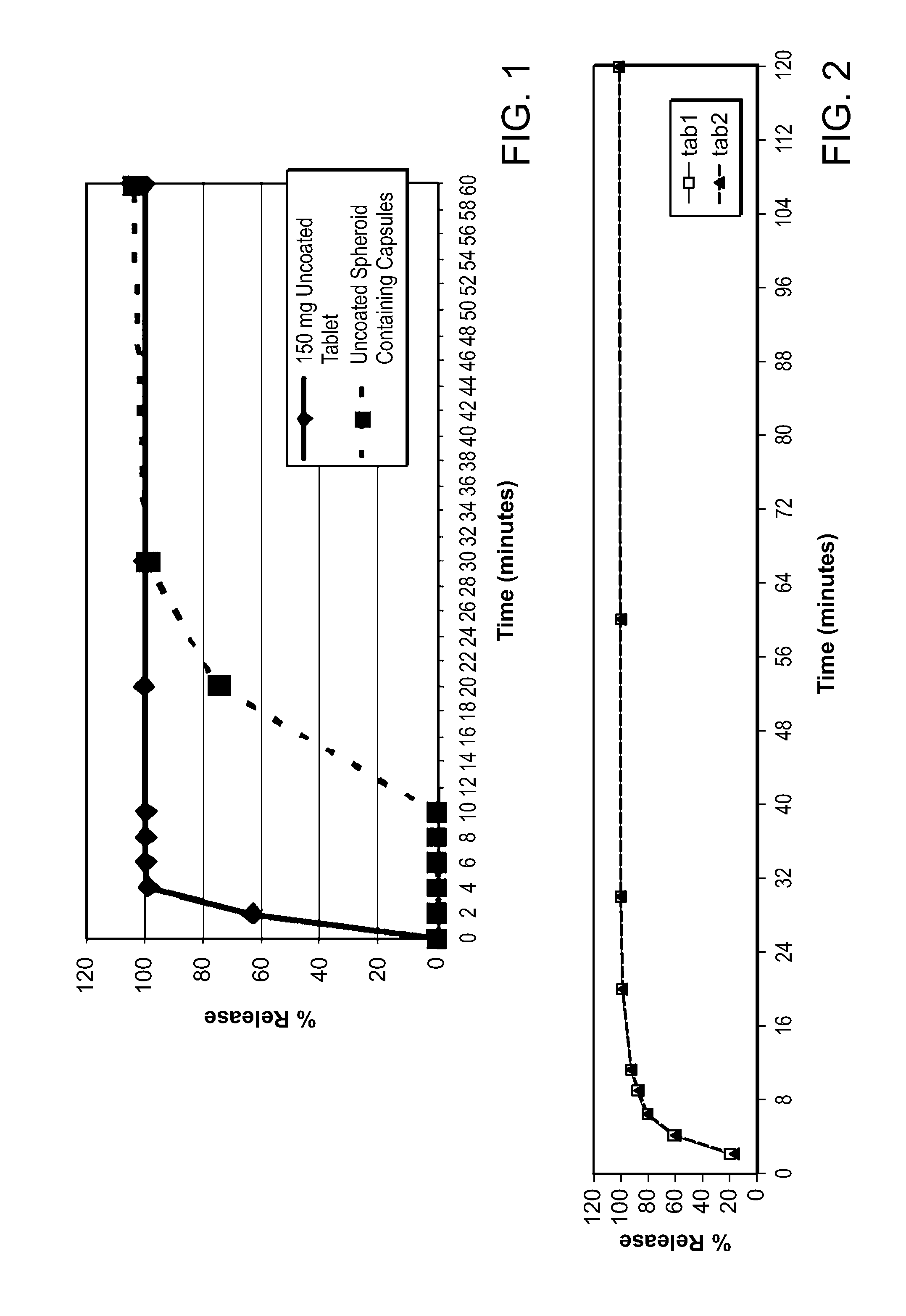
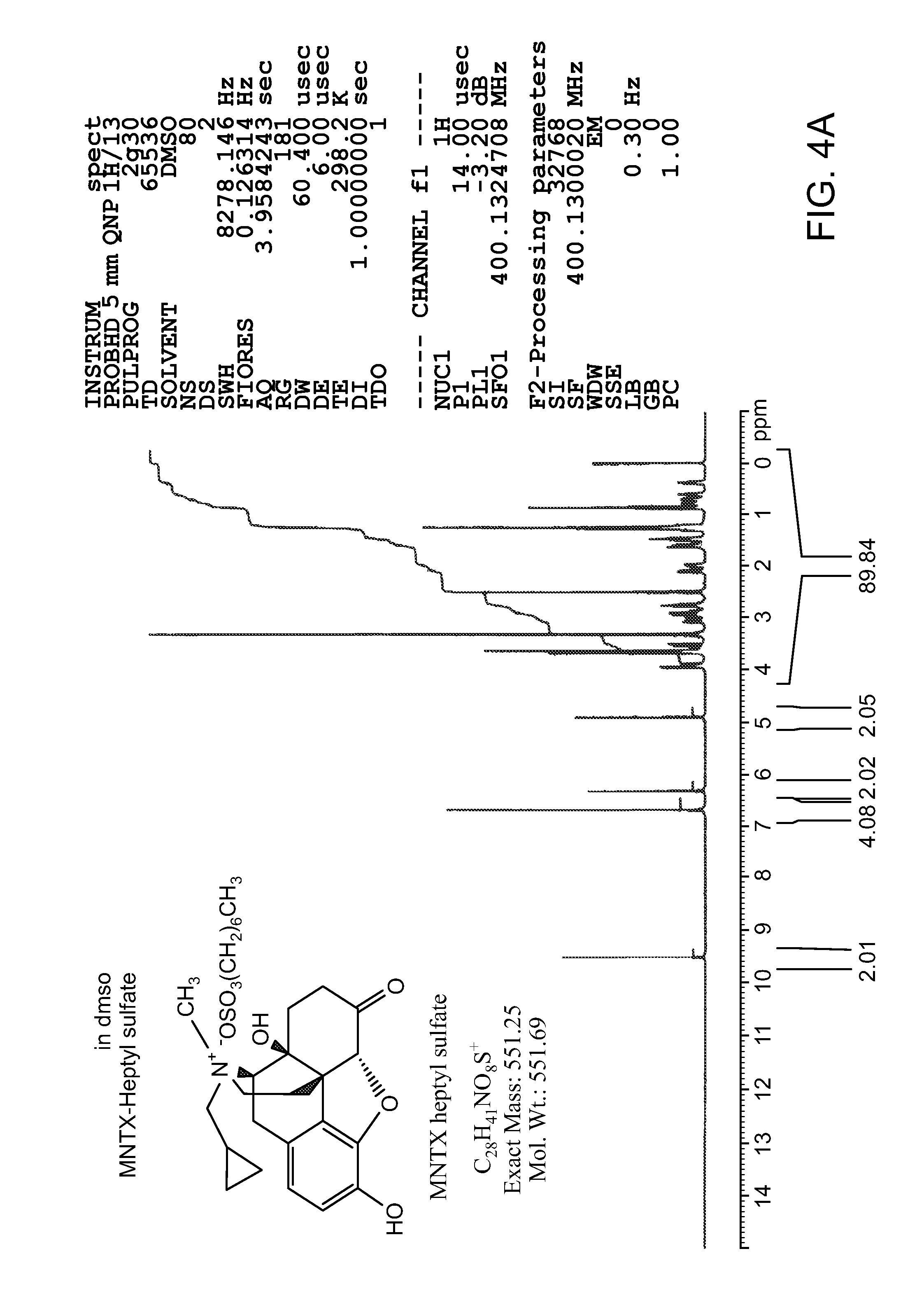
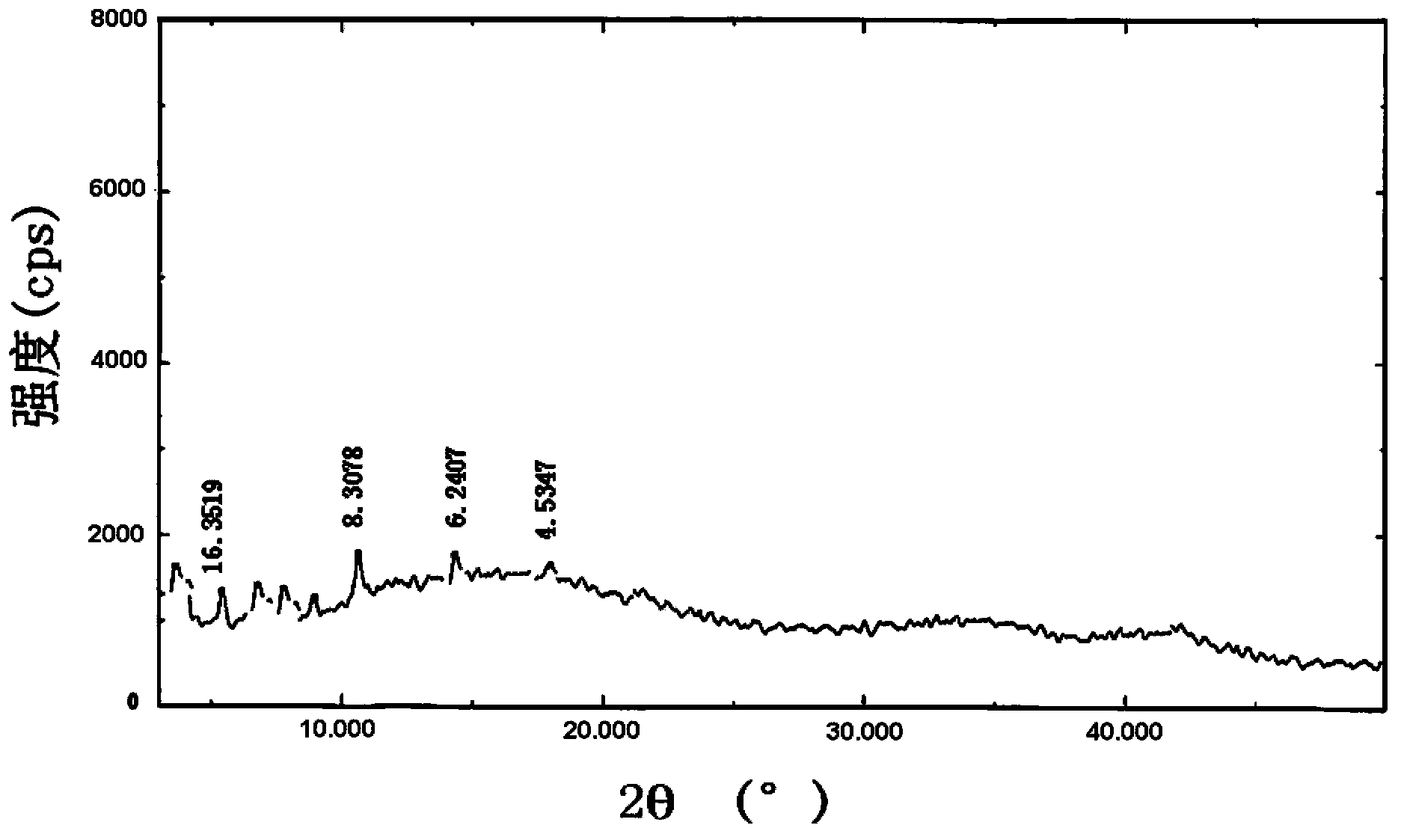
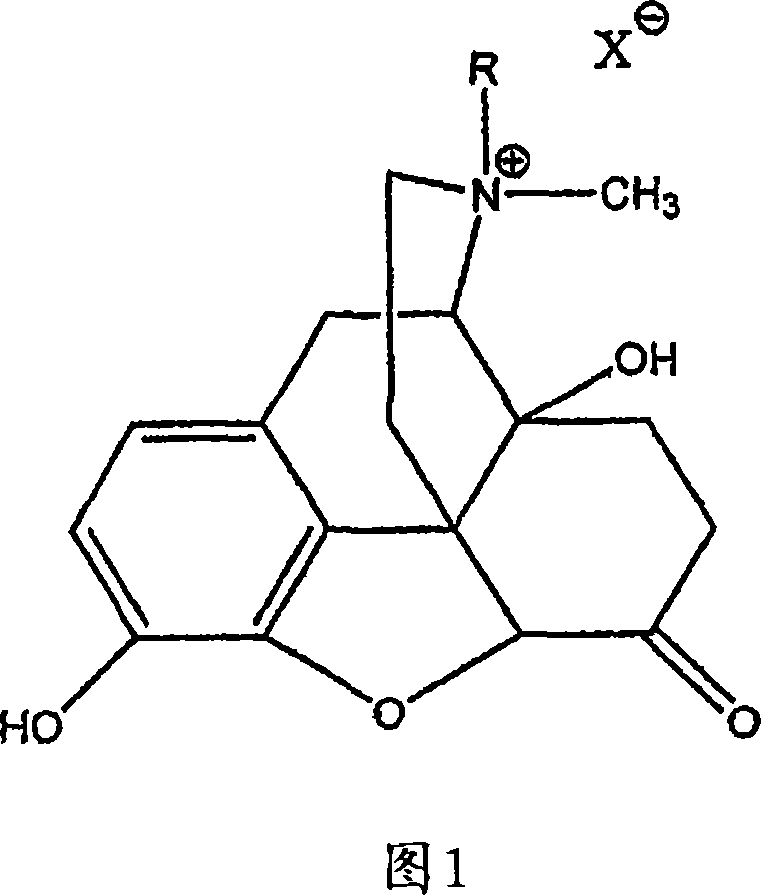
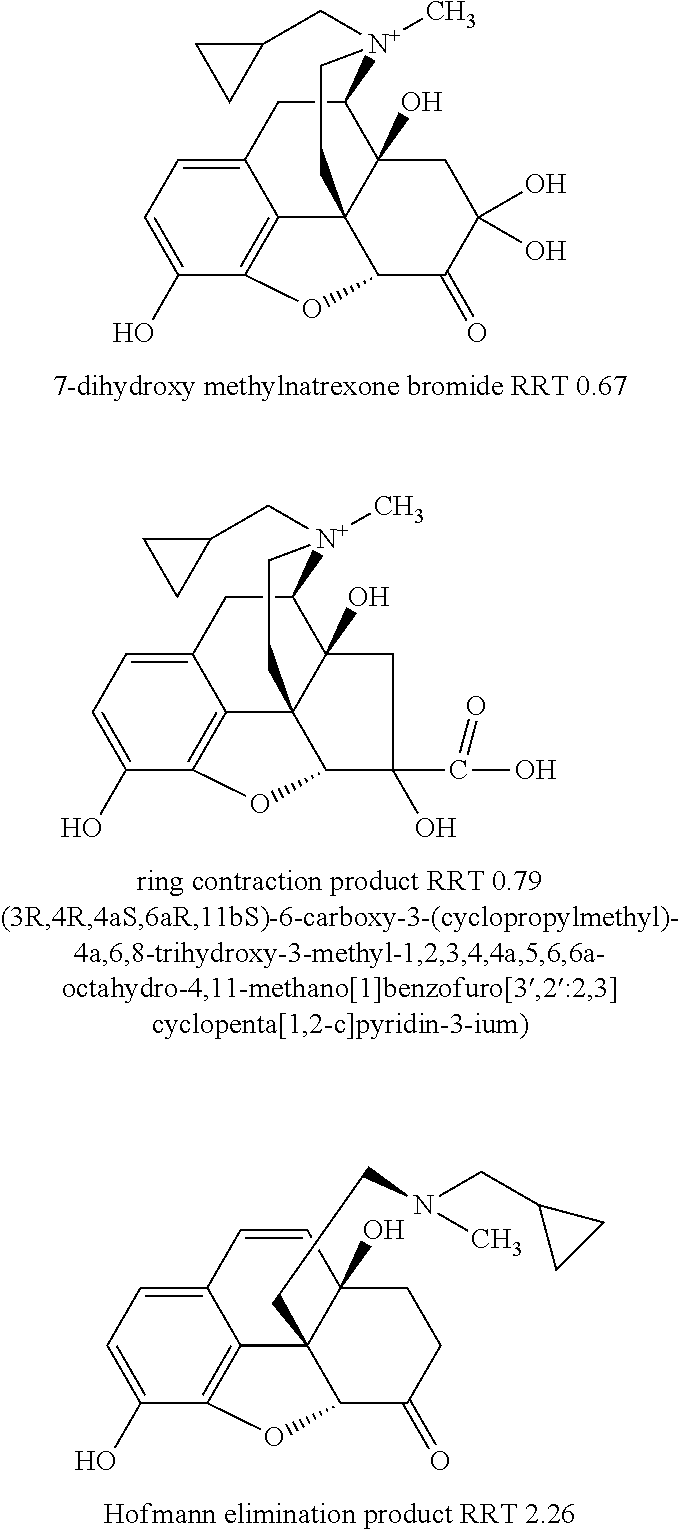
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
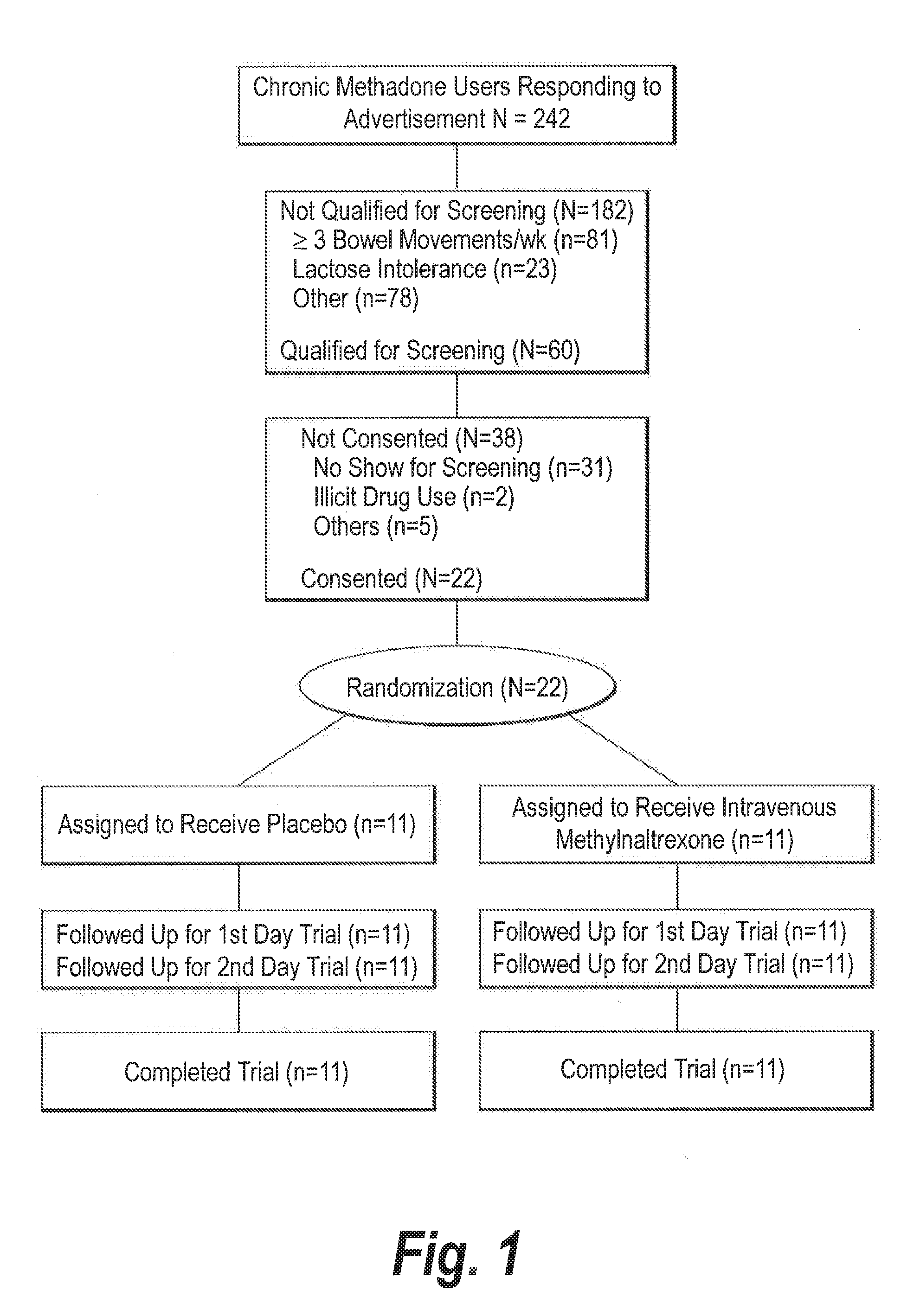
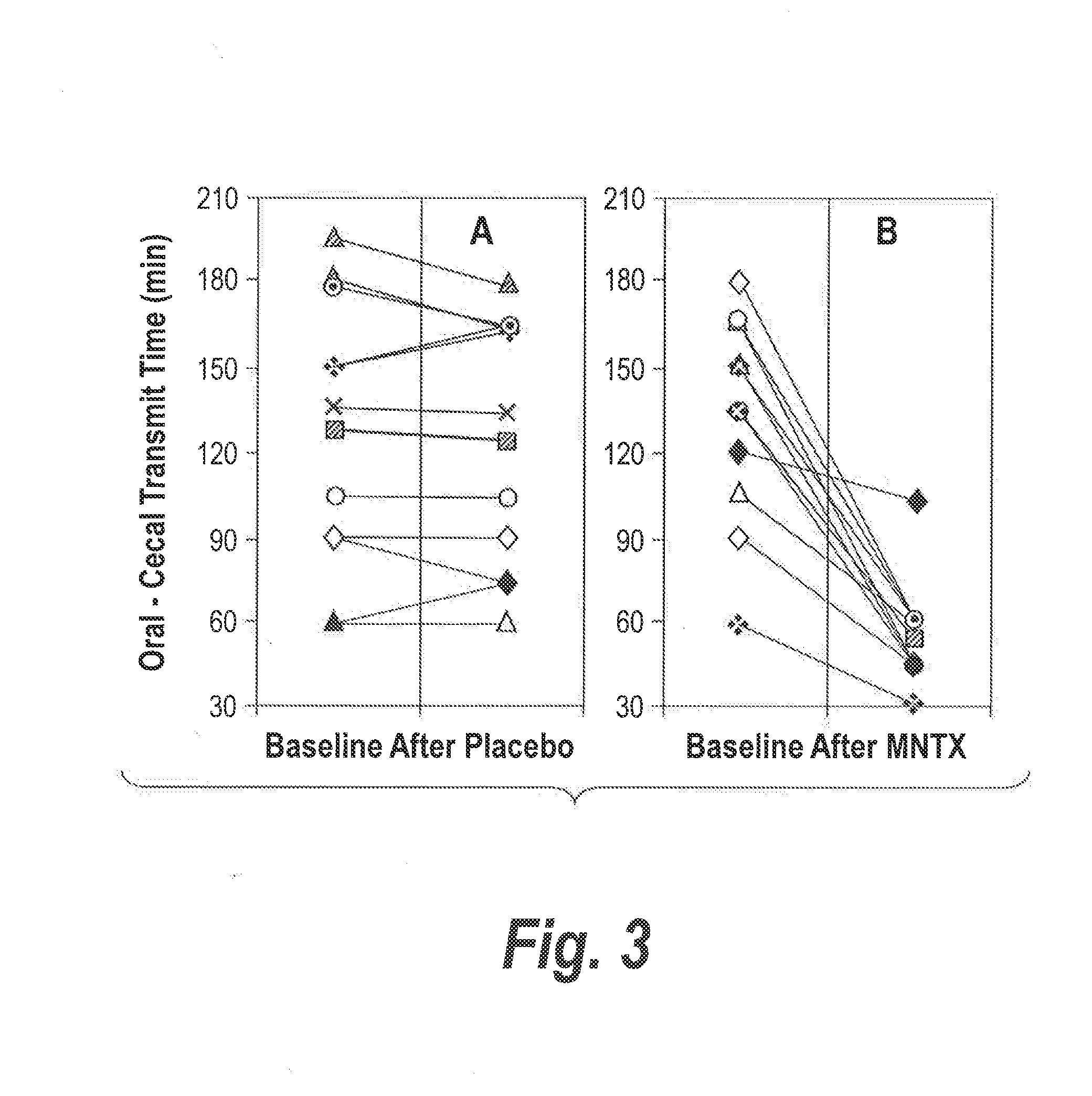
46 results about "Methylnaltrexone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat constipation caused by opioid (narcotic) medication.
Use of methylnaltrexone to treat irritable bowel syndrome
Methods of treating irritable bowel syndrome with peripheral opioid antagonists, such as methylnaltrexone, are provided. Formulations comprising peripheral opioid antagonists, such as methylnaltrexone, and irritable bowel syndrome therapeutic agents are also provided.
Owner:PROGENICS PHARMA INC
Dry powder compound formulations and uses thereof
InactiveUS20080064743A1Improve angiogenesisPreventing, treatingBiocideNervous disorderSide effectCompound (substance)
The present invention provides lyophilized formulations comprising methylnaltrexone, and processes for preparation of provided formulations. Additionally provided are compositions and products containing the methylnaltrexone formulation, as well as methods for producing formulations, compositions and products. Provided formulations as well as compositions and products containing methylnaltrexone formulations are useful for preventing, treating delaying, diminishing or reducing the severity and / or incidence of side effects resulting from administration of analgesic opioids.
Owner:WYETH LLC
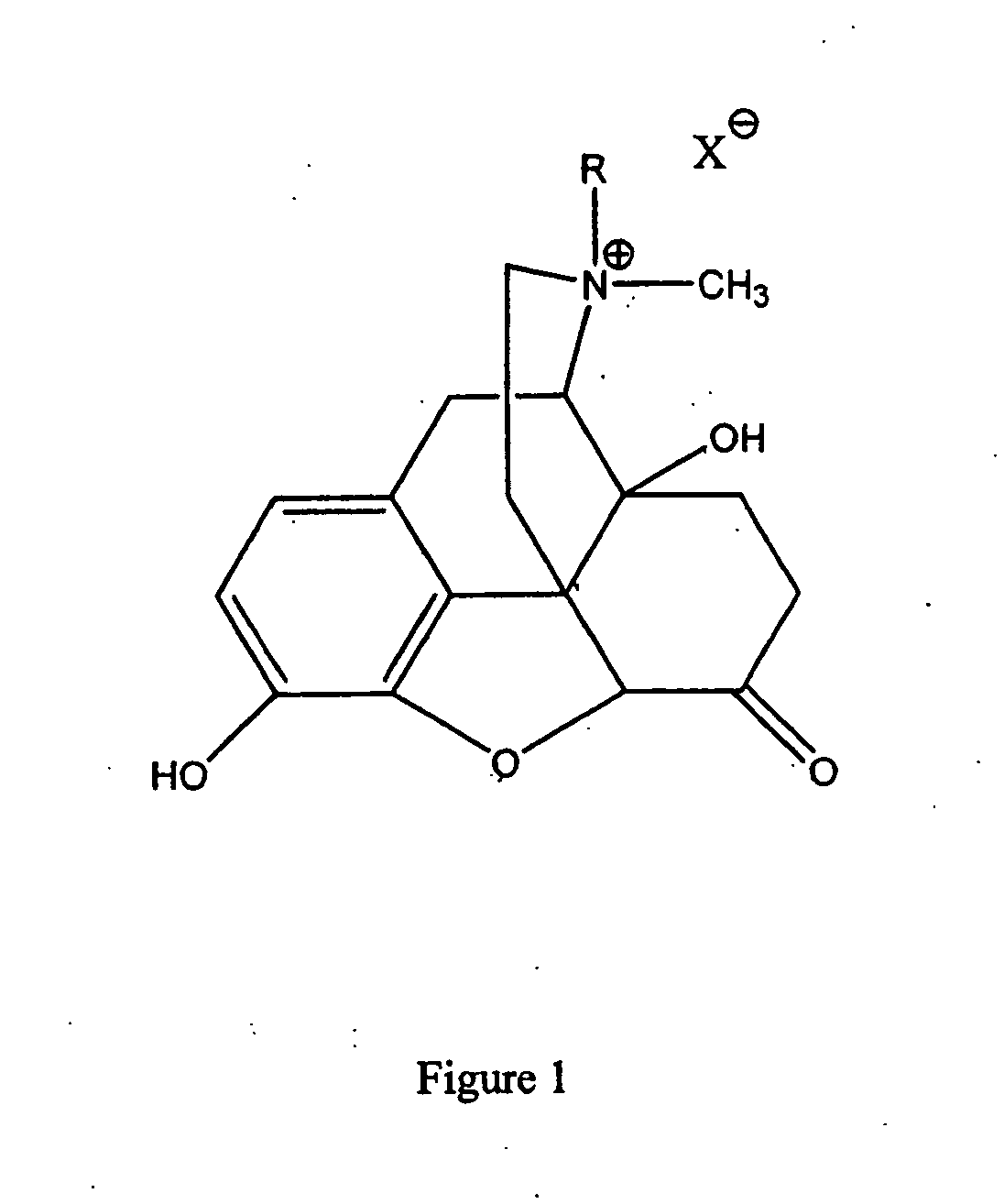
(S)-N-methylnaltrexone
Owner:PROGENICS PHARMA INC
Synthesis of R-N-methylnaltrexone
InactiveUS20070099946A1Organic active ingredientsAntipyreticCombinatorial chemistryStereoselectivity
Owner:PROGENICS PHARMA INC
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
Formulations for parenteral delivery of compounds and uses thereof
The present invention provides formulations that achieve effective delivery of methylnaltrexone compositions. The provided formulations are useful for preventing, treating delaying, diminishing or reducing the severity of side effects resulting from use of analgesic opioids.
Owner:WYETH LLC
Use of methylnaltrexone and related compounds
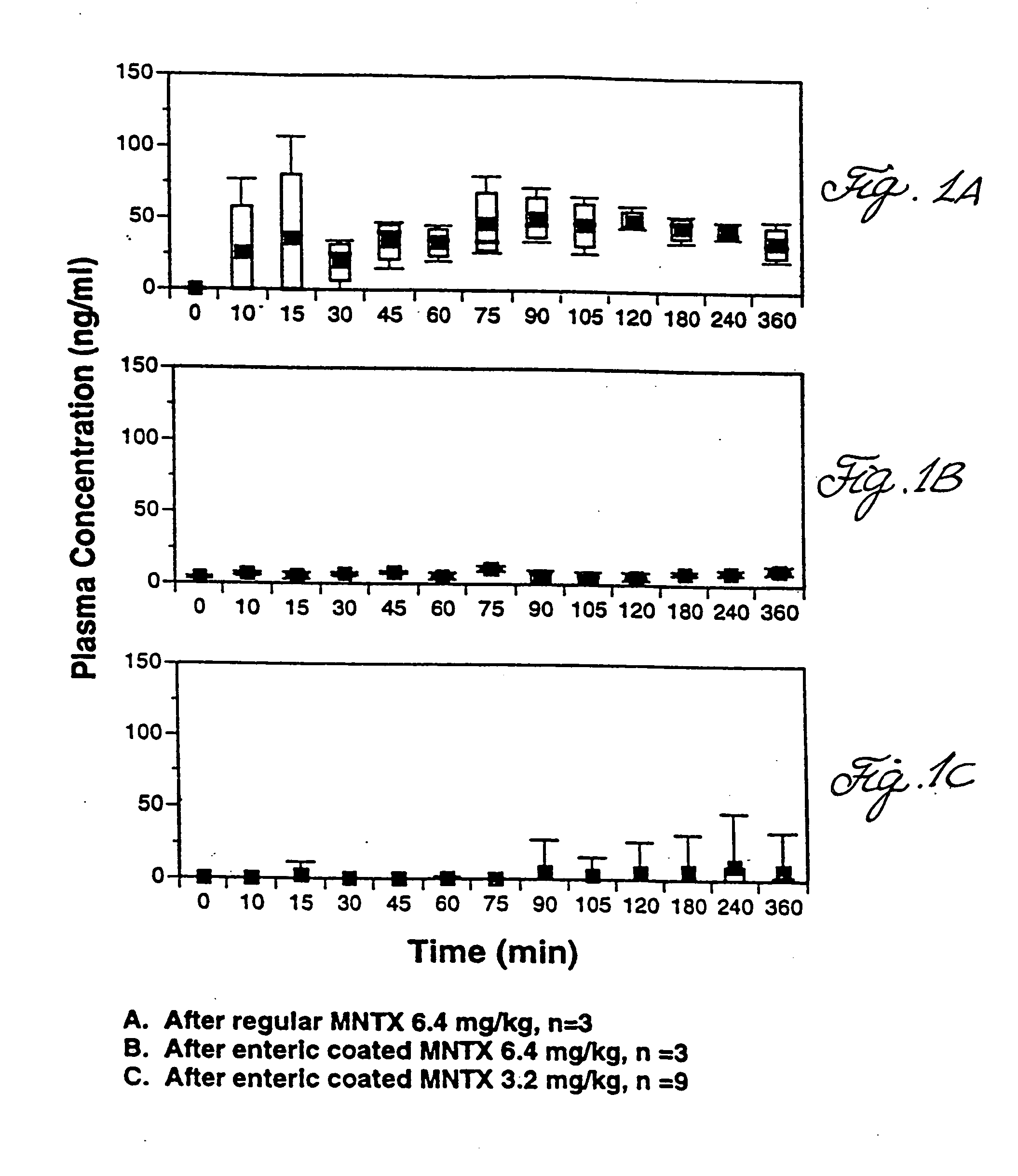
A composition for preventing or treating the opioid induced side effect, inhibition of gastrointestinal motility is disclosed. The composition comprises methylnaltrexone or another quaternary derivative of noroxymorphone administered to a patient prior to the administration of an opioid or after the onset of side effects induced by the administration of an opioid, wherein the methylnaltrexone or quaternary derivative is administered orally in an enterically coated form.
Owner:PROGENICS PHARMA NEVADA
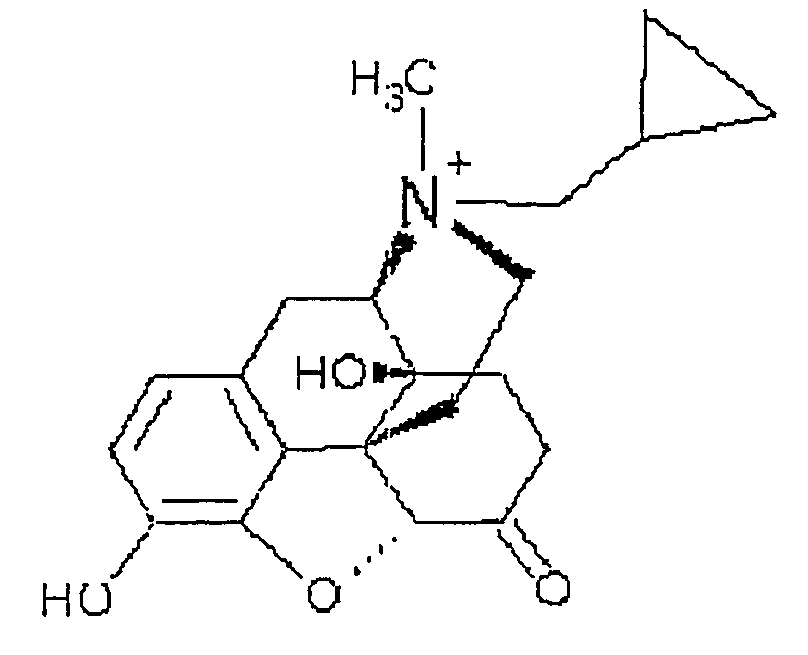
Process and compounds for the production of (+)opiates
The invention generally provides processes and intermediate compounds useful for the production of (+)-opiates. Non-limiting examples of (+) opiates that may be derived from one or more compounds of the invention include (+)-noroxymorphone, (+)-naltrexone, (+)-naloxone, (+)-N-cyclopropylmethylnorhydrocodone, (+)-N-cycloproylmethylnorhydromorphone, (+)-N-allylnorhydrocodone, (+)-N-allylnorhydromorphone, (+)-noroxycodone, (+)-naltrexol, (+)-naloxol, and (+)-3-O-methyl-naltrexone.
Owner:SPECGX LLC
Synthesis of R-N-methylnaltrexone
Owner:PROGENICS PHARMA INC
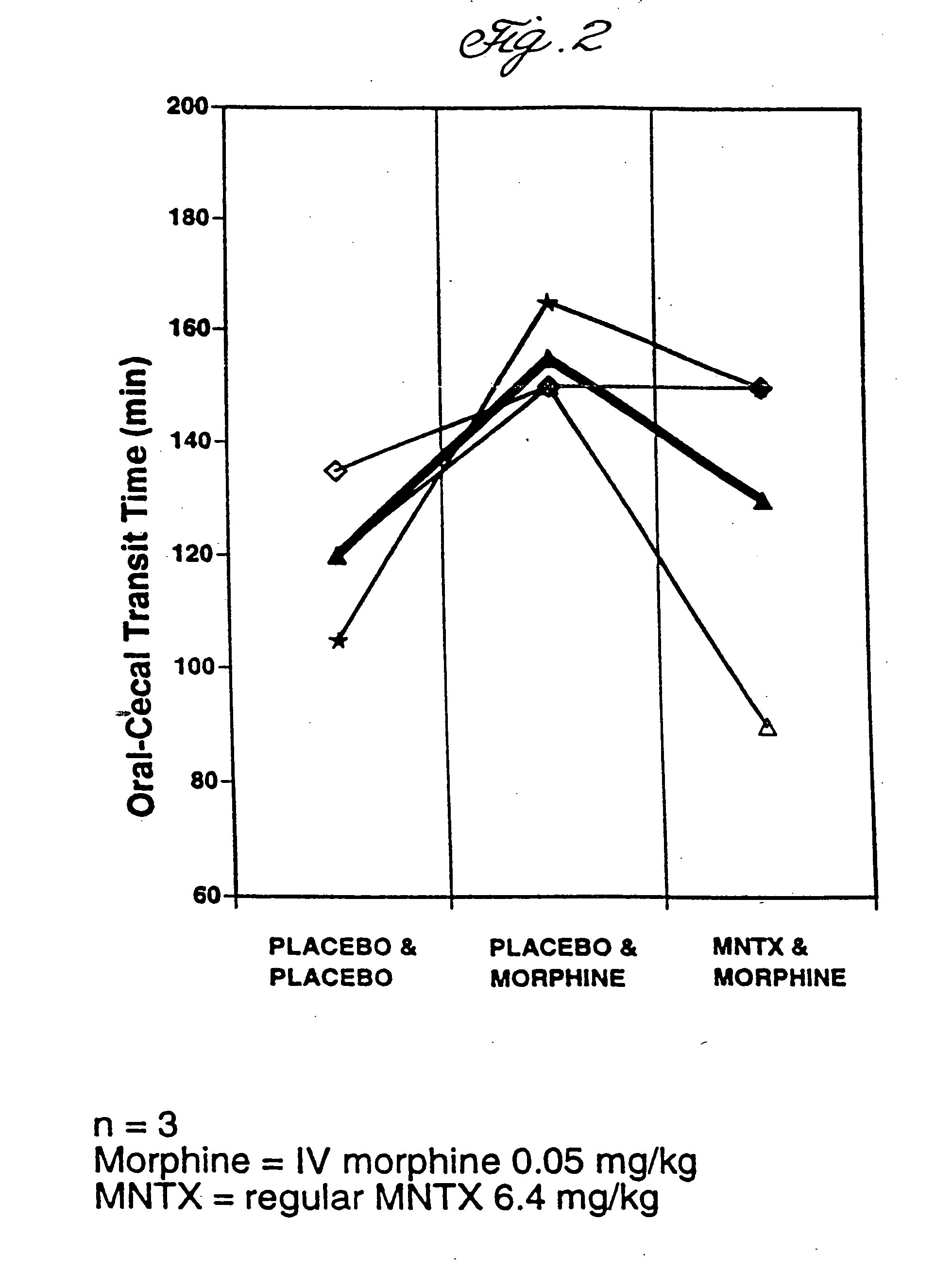
Use of methylnaltrexone and related compounds to treat post-operative gastrointestinal dysfunction
InactiveUS20060205753A1Restoring gastrointestinal activityPromote recoveryBiocideDigestive systemSegmental colectomyBowel dysfunction
Methods and compositions for treating post-surgical gastrointestinal dysfunction are provided. Methods include administering a quaternary derivative of noroxymorphone (e.g., methylnaltrexone) to a patient after a segmental colectomy is performed on the patient.
Owner:PROGENICS PHARMA INC
Use of methylnaltrexone in treating gastrointestinal dysfunction in equines
InactiveUS20050011468A1Relieving inhibition of gastrointestinal motilityMaintaining pain-reducing effectOrganic active ingredientsOther apparatusMotilityEquine Species
Systems and methods are described for using methylnaltrexone to treat or prevent inhibition of gastrointestinal motility in equines. A method for preventing or treating opioid-induced and non-opioid-induced gastrointestinal dysfunction includes administering a quaternary derivative of noroxymorphone, preferably methylnaltrexone, to an equine before or after the onset of the gastrointestinal dysfunction.
Owner:PROGENICS PHARMA INC
Use of methylnaltrexone and related compound to treat constipation in chronic opioid users
InactiveUS20100087472A1Improve the quality of lifeMaintains analgesic efficacyBiocideDigestive systemSide effectMedicine
A method of preventing or treating constipation in a patient who has been chronically taking opioids, the method comprising administering a quaternary derivative of noroxymorphone in an amount sufficient to prevent or treat the side effect in the patient, but which amount would be insufficient to treat a patient with the same opioid-induced side effect who had not chronically been administered opioids.
Owner:PROGENICS PHARMA NEVADA +1
(S)-N-methylnaltrexone
Owner:PROGENICS PHARMA INC
Use of methylnaltrexone and related compounds for treatment of gastrointestinal dysfunction induced by endogenous opioids
Owner:FOSS JOSEPH F +4
Formulations for parenteral delivery of compounds and uses thereof
The present invention provides formulations that achieve effective delivery of methylnaltrexone compositions. The provided formulations are useful for preventing, treating delaying, diminishing or reducing the severity of side effects resulting from use of analgesic opioids.
Owner:WYETH LLC
Pharmaceutical formulation
InactiveUS20100261746A1Eliminate side effectsReduce secretionBiocideNervous disorderPharmaceutical formulationMethylnaltrexone
Stable pharmaceutical compositions useful for administering methylnaltrexone are described, as are methods for making the same. Kits, including these pharmaceutical compositions, also are provided.
Owner:PROGENICS PHARMA INC
Pharmaceutical formulation
InactiveUS20100267758A1Delayed gastric emptyingReduce secretionBiocideNervous disorderPharmaceutical formulationDrug formulations
Stable pharmaceutical compositions useful for administering methylnaltrexone are described, as are methods for making the same. Kits, including these pharmaceutical compositions, also are provided.
Owner:PROGENICS PHARMA INC
Pharmaceutical formulation
InactiveUS20100261745A1Eliminate side effectsReduce secretionBiocideNervous disorderPharmaceutical formulationMethylnaltrexone
Stable pharmaceutical compositions useful for administering methylnaltrexone are described, as are methods for making the same. Kits, including these pharmaceutical compositions, also are provided.
Owner:PROGENICS PHARMA INC
Stable methylnaltrexone preparation
InactiveUS8552025B2Delayed gastric emptyingReduce secretionBiocideNervous disorderMethylnaltrexoneDrug
Stable pharmaceutical compositions useful for administering methylnaltrexone are described, as are methods for making the same. Kits, including these pharmaceutical compositions, also are provided.
Owner:PROGENICS PHARMA INC
Pharmaceutical formulation
ActiveUS20100261744A1Eliminate side effectsReduce secretionBiocideNervous disorderPharmaceutical formulationMethylnaltrexone
Stable pharmaceutical compositions useful for administering methylnaltrexone are described, as are methods for making the same. Kits, including these pharmaceutical compositions, also are provided.
Owner:PROGENICS PHARMA INC
Oral formulations and lipophilic salts of methylnaltrexone
ActiveUS20120070495A1Promote absorptionImprove usabilityBiocideNervous disorderOral medicationMethylnaltrexone
The present invention provides compositions comprising methylnaltrexone or a salt thereof, and compositions and formulations thereof, for oral administration.
Owner:WYETH LLC
Oral formulations and lipophilic salts of methylnaltrexone
ActiveUS8524276B2Promote absorptionReduce severityBiocideNervous disorderOral medicationOral administration
The present invention provides compositions comprising methylnaltrexone or a salt thereof, and compositions and formulations thereof, for oral administration.
Owner:WYETH LLC
Use of methylnaltrexone and related compounds to treat constipation in chronic opioid users
InactiveUS20120190702A1Improve the quality of lifeEffect andBiocideDigestive systemSide effectOpioid use
A method of preventing or treating constipation in a patient who has been chronically taking opioids, the method comprising administering a quaternary derivative of noroxymorphone in an amount sufficient to prevent or treat the side effect in the patient, but which amount would be insufficient to treat a patient with the same opioid-induced side effect who had not chronically been administered opioids.
Owner:UNIVERSITY OF CHICAGO
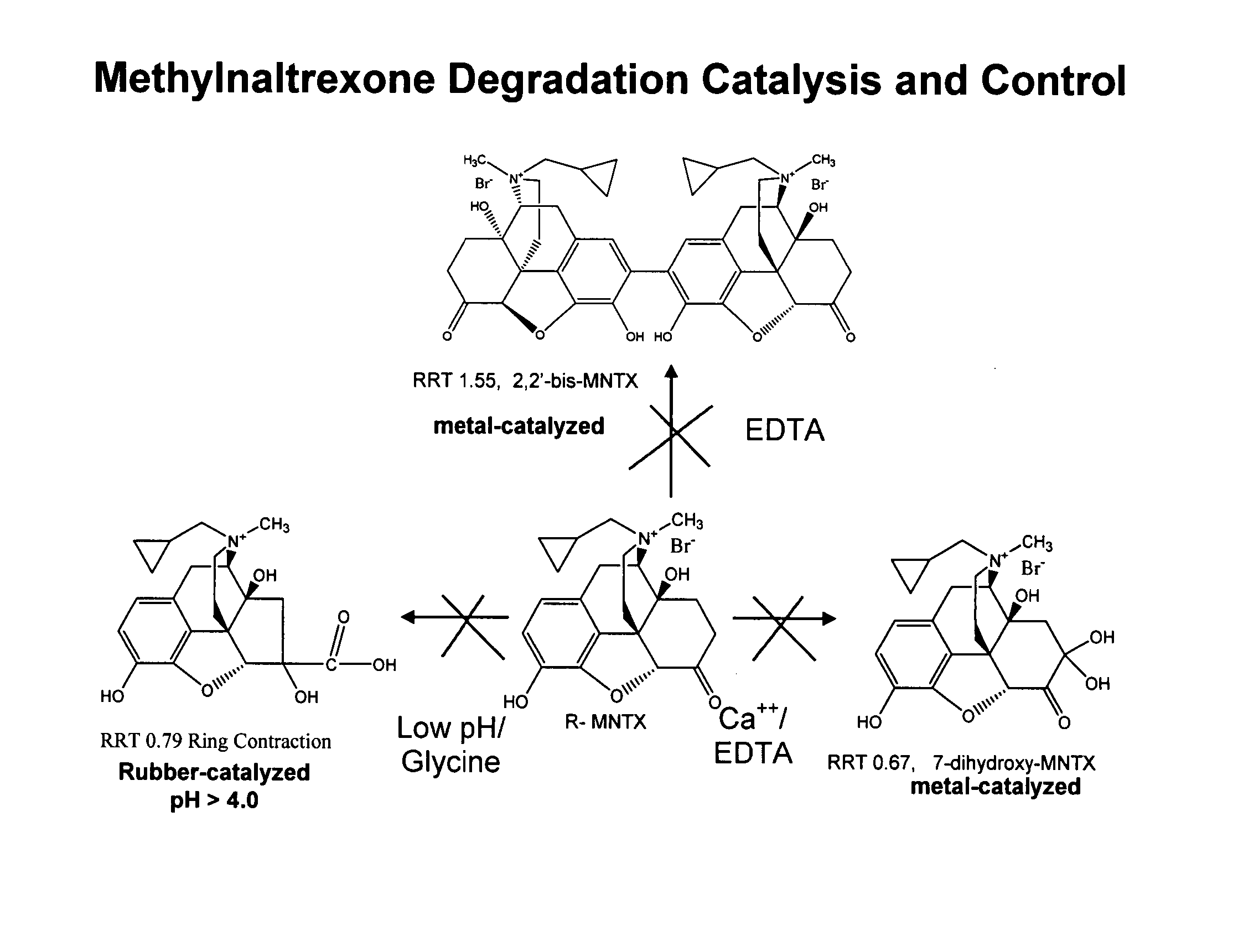
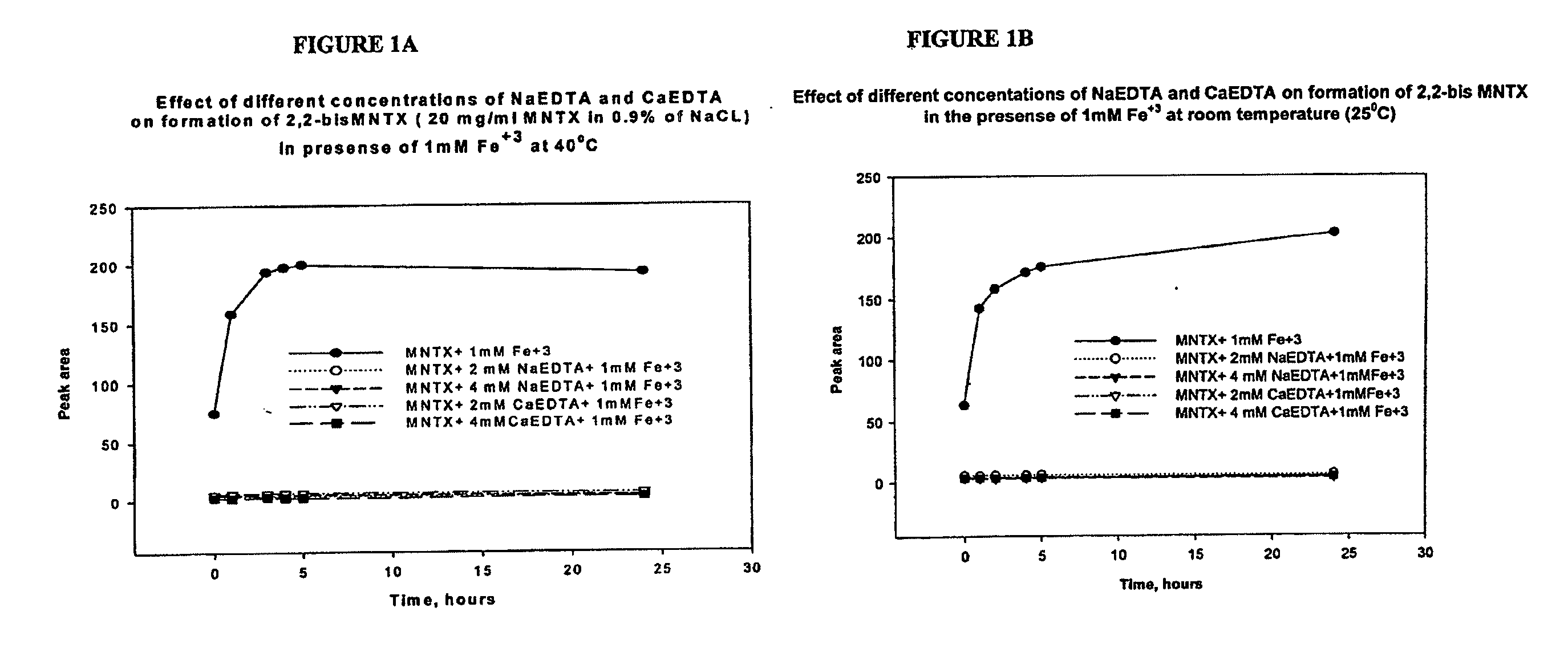
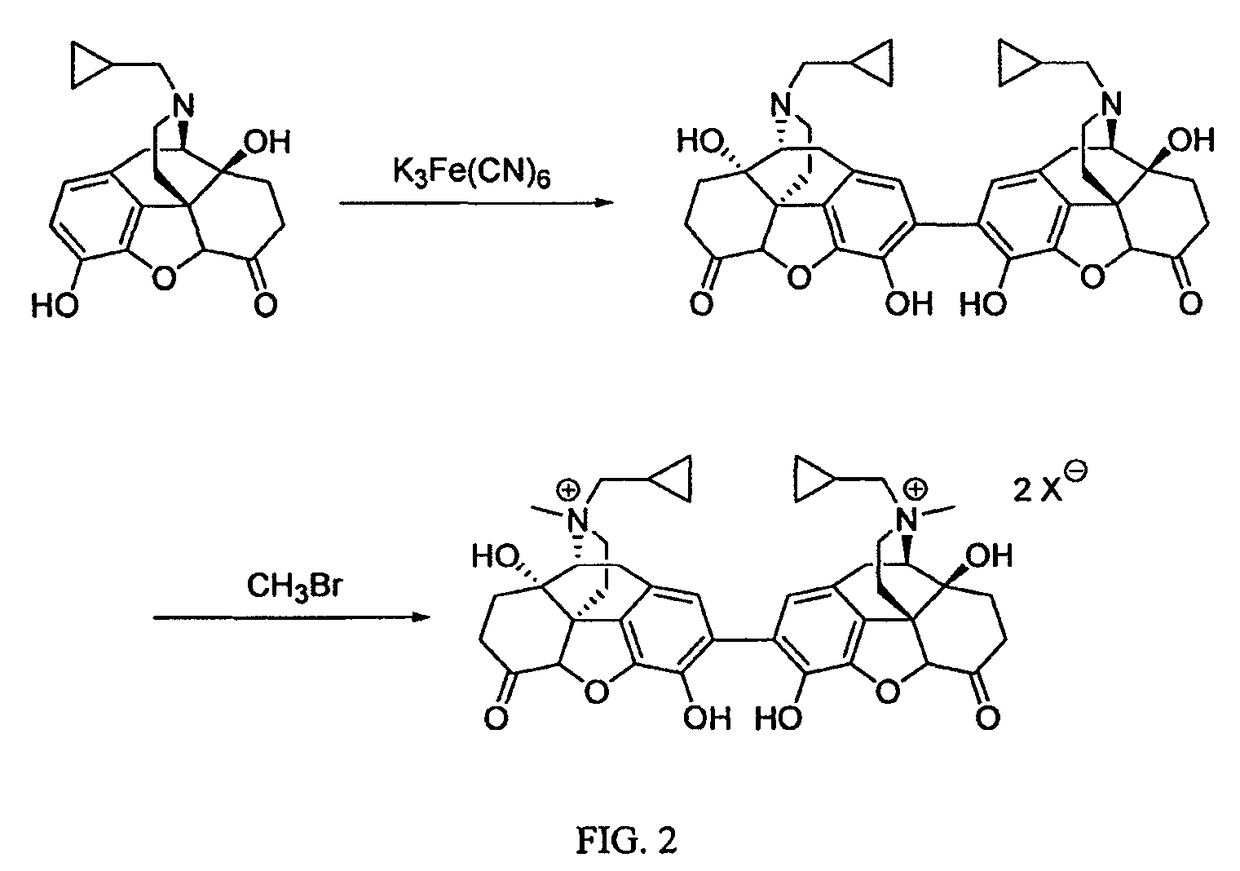
Preparation and use of (R),(R)-2,2′-bis-methylnaltrexone
Owner:PROGENICS PHARMA INC
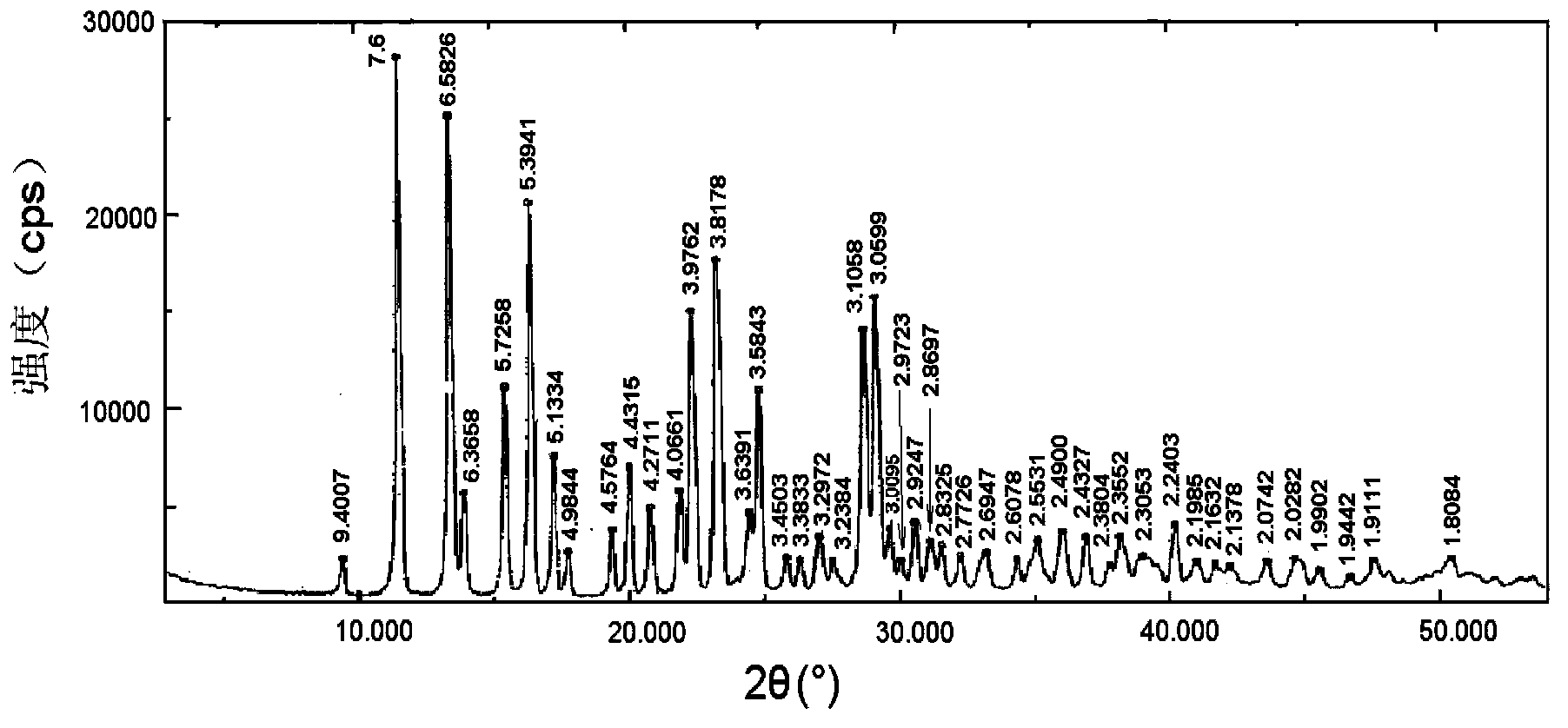
Methylnaltrexone compound, oral tablet and preparation methods of two
ActiveCN104274414AIncrease fat solubilityPromote oral absorptionOrganic active ingredientsDigestive systemAdhesiveEvaporation
The invention provides a methylnaltrexone compound. The compound comprises methylnaltrexone or pharmaceutically-acceptable salts and phosphatide, wherein the molar ratio of methylnaltrexone or the pharmaceutically-acceptable salts to phosphatide is 1:0.5-2, and the compound is prepared by dissolving methylnaltrexone or the pharmaceutically-acceptable salts and phosphatide in a solvent and performing evaporation to remove the solvent. The invention also provides a methylnaltrexone oral tablet. The oral table contains the above methylnaltrexone compound and additives, and the additives are one or more selected from fillers, disintegrating agents, adhesives, chelating agents, stabilizing agents and lubricants. A preparation method of the oral tablet comprises the following steps: mixing the methylnaltrexone compound with the additives and tabletting to prepare the methylnaltrexone oral tablet.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Use of methylnaltrexone and related compounds to treat post-operative gastrointestinal dysfunction
Methods and compositions for treating post-surgical gastrointestinal dysfunction are provided. Methods include administering a quaternary derivative of noroxymorphone (e.g., methylnaltrexone) to a patient after a segmental colectomy is performed on the patient.
Owner:PROGENICS PHARMA INC
Dry powder compound formulations and uses thereof
InactiveUS20120059025A1Improve angiogenesisPreventing, treatingBiocideNervous disorderSide effectChemical compound
The present invention provides lyophilized formulations comprising methylnaltrexone, and processes for preparation of provided formulations. Additionally provided are compositions and products containing the methylnaltrexone formulation, as well as methods for producing formulations, compositions and products. Provided formulations as well as compositions and products containing methylnaltrexone formulations are useful for preventing, treating delaying, diminishing or reducing the severity and / or incidence of side effects resulting from administration of analgesic opioids.
Owner:WYETH LLC
Methods for the treatment or inhibition of ileus
Methods for the treatment of ileus are disclosed wherein the peripheral mu opioid antagonist methylnaltrexone is administered.
Owner:MERCK SHARP & DOHME LLC
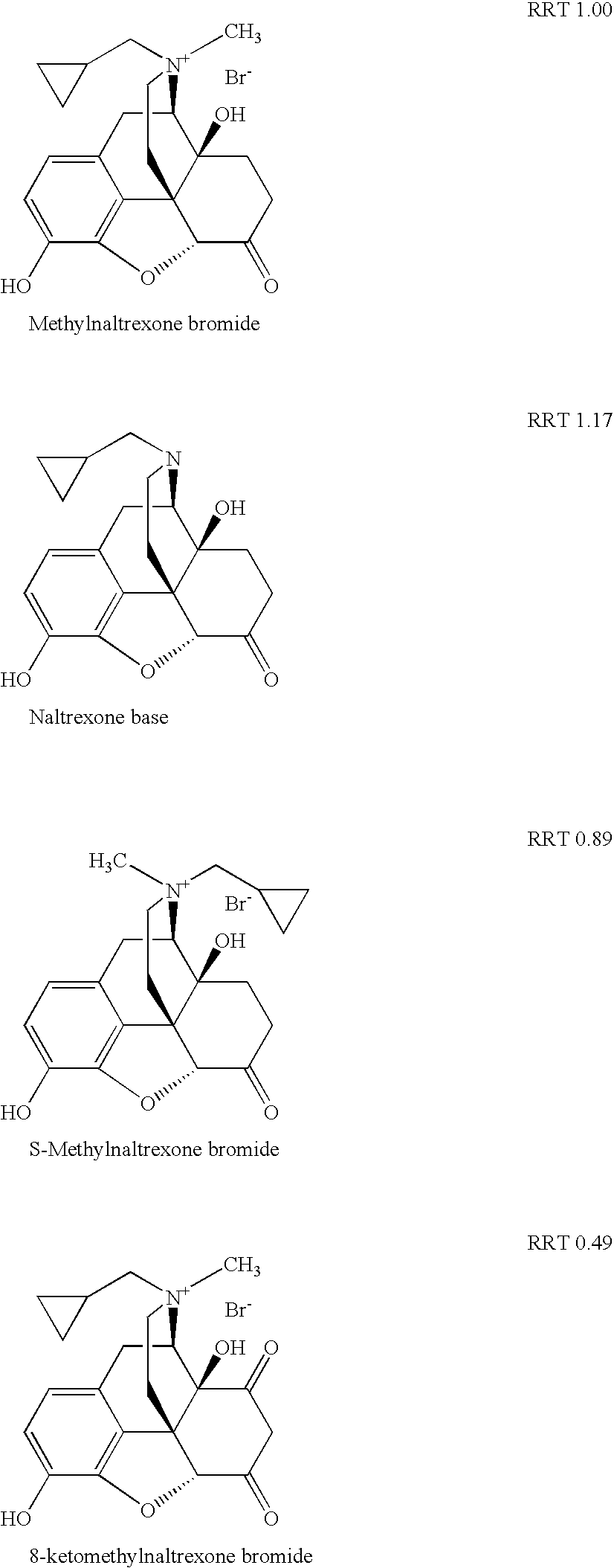
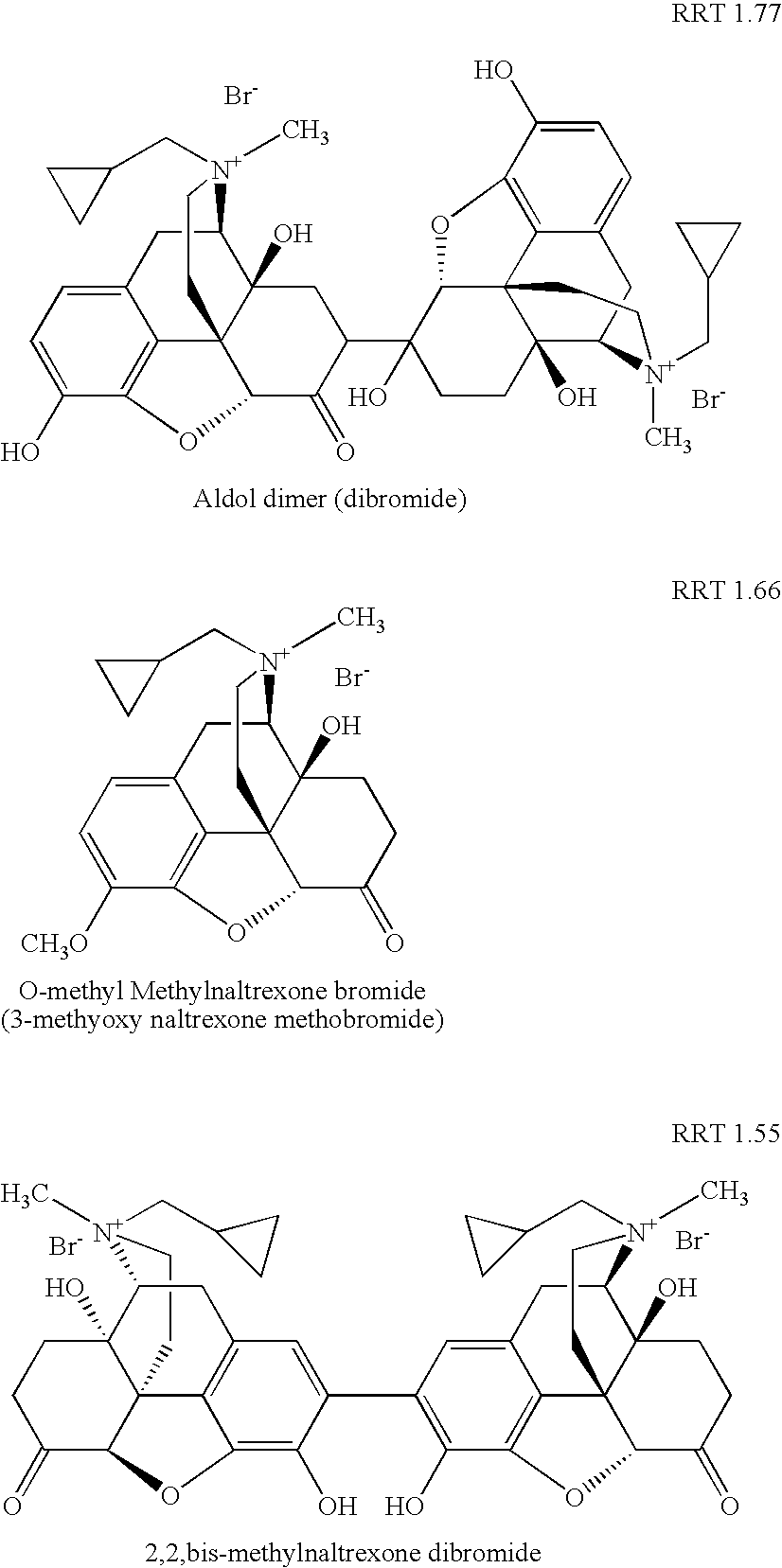
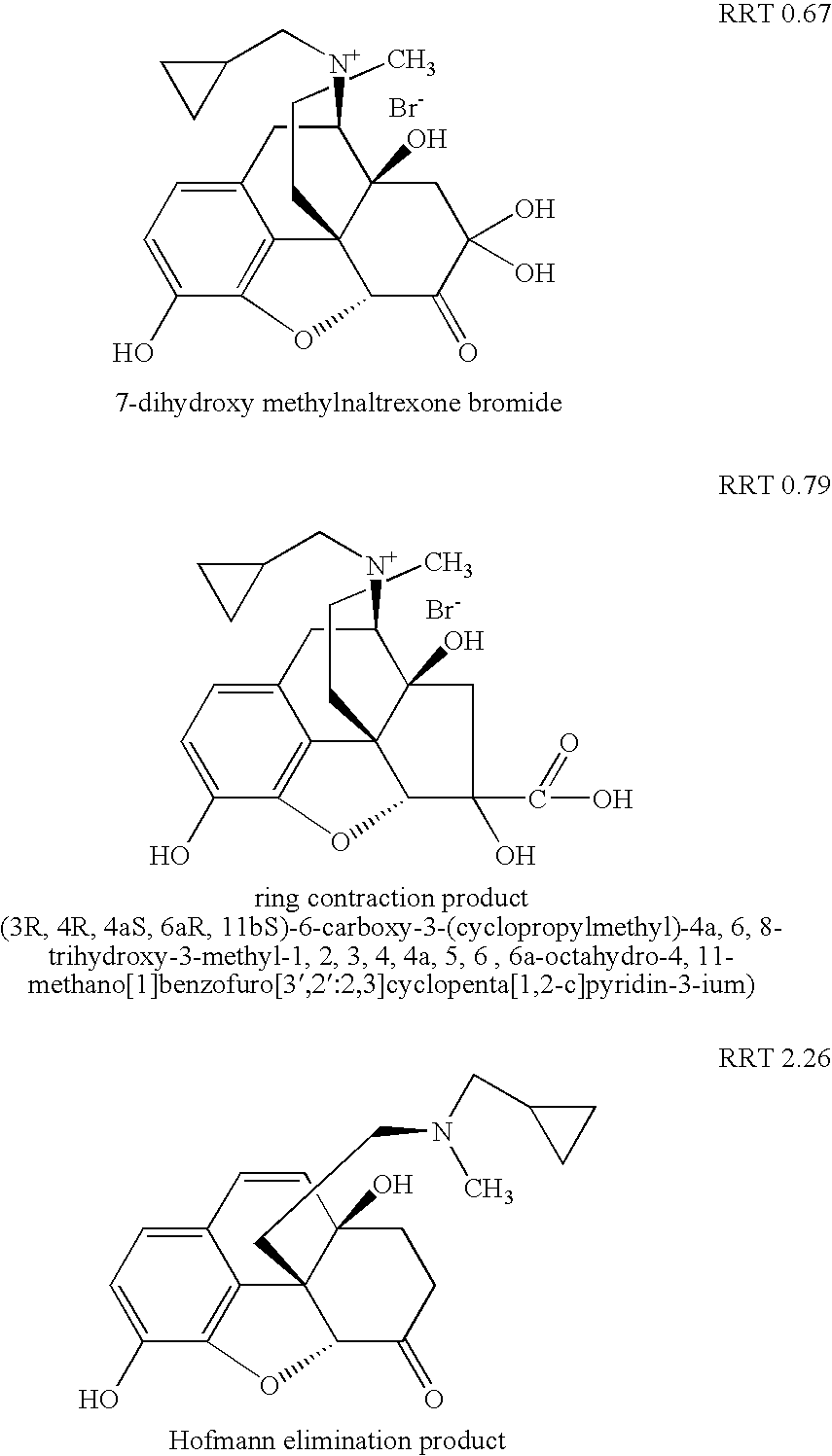
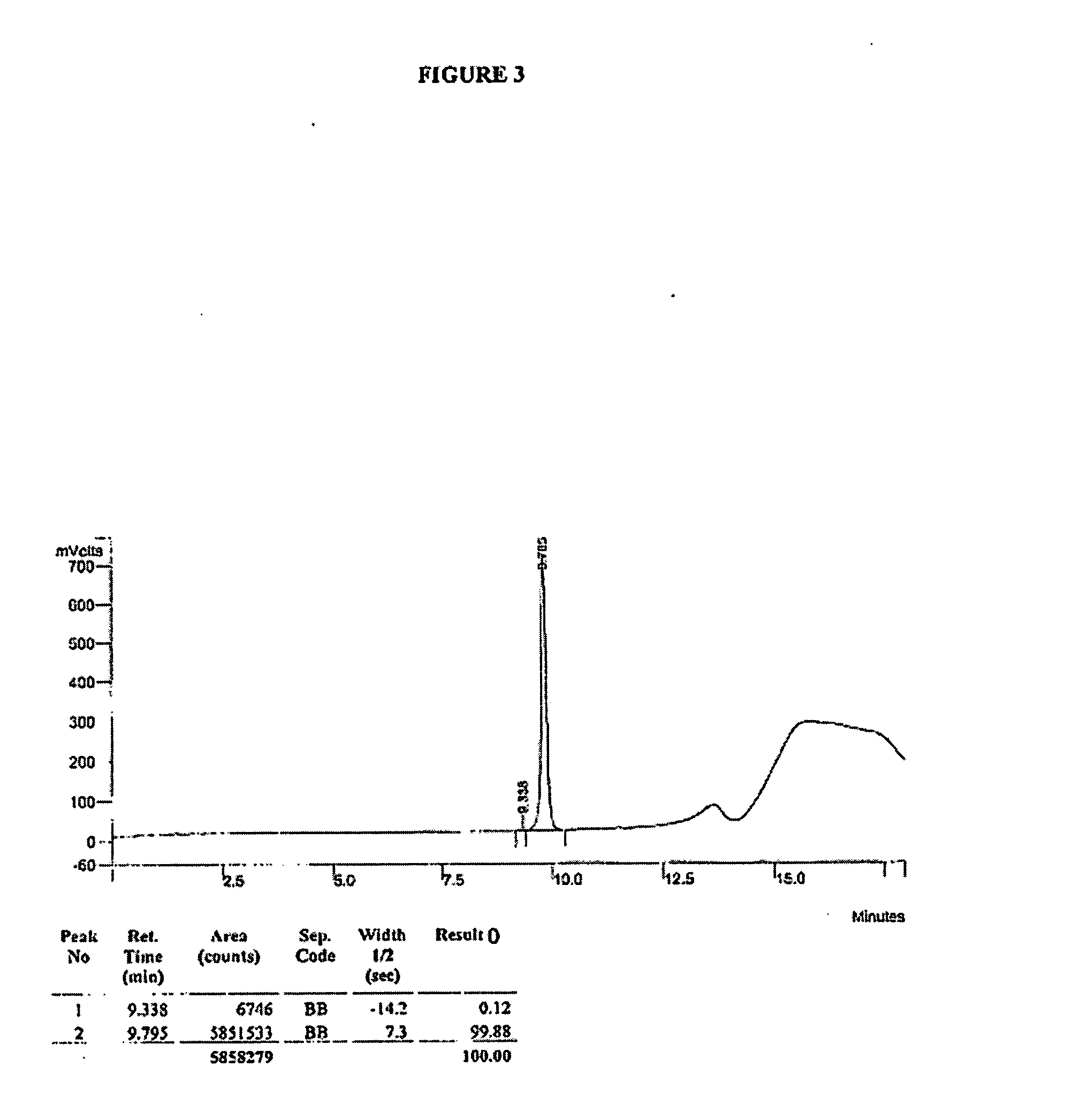
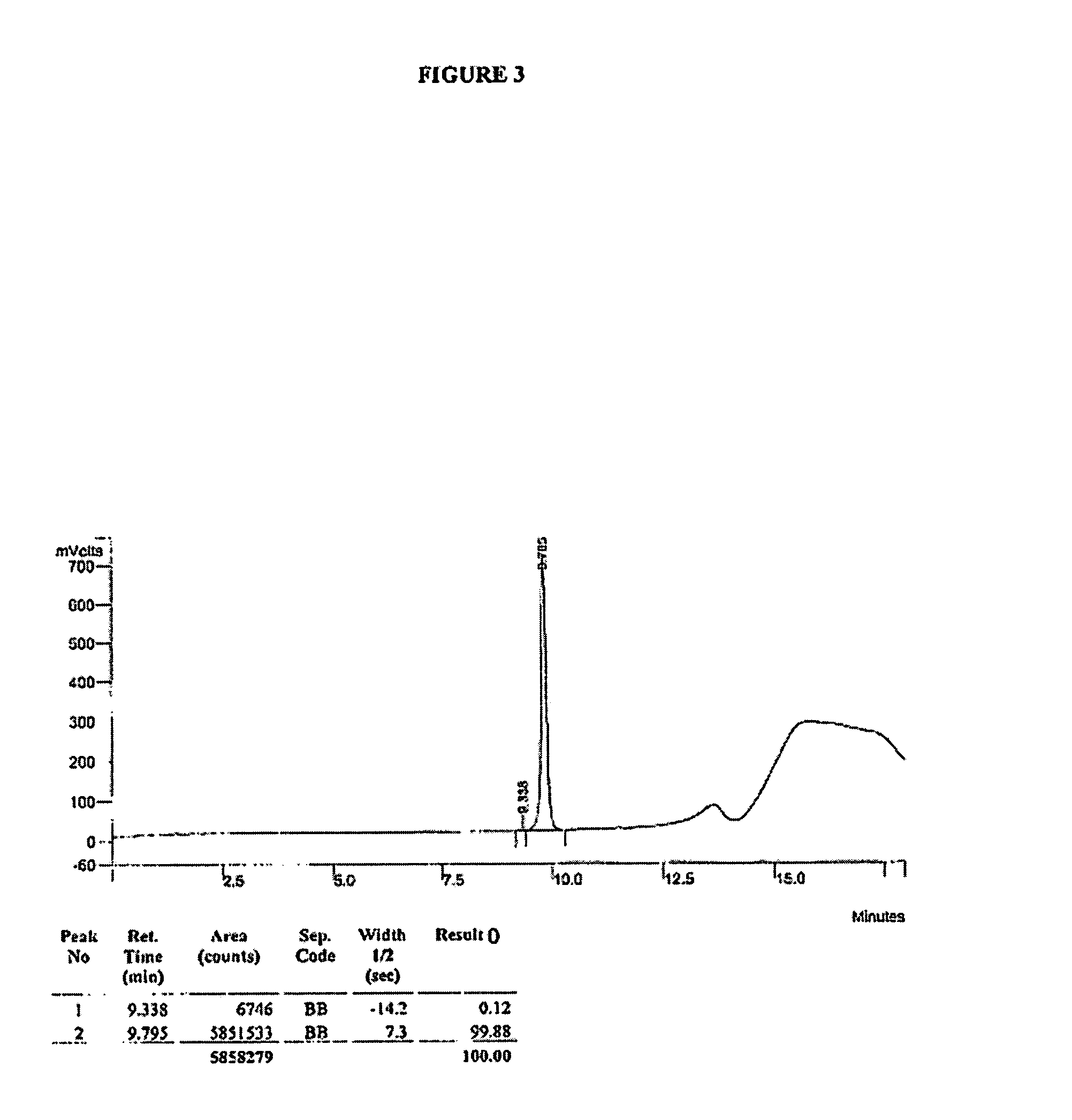
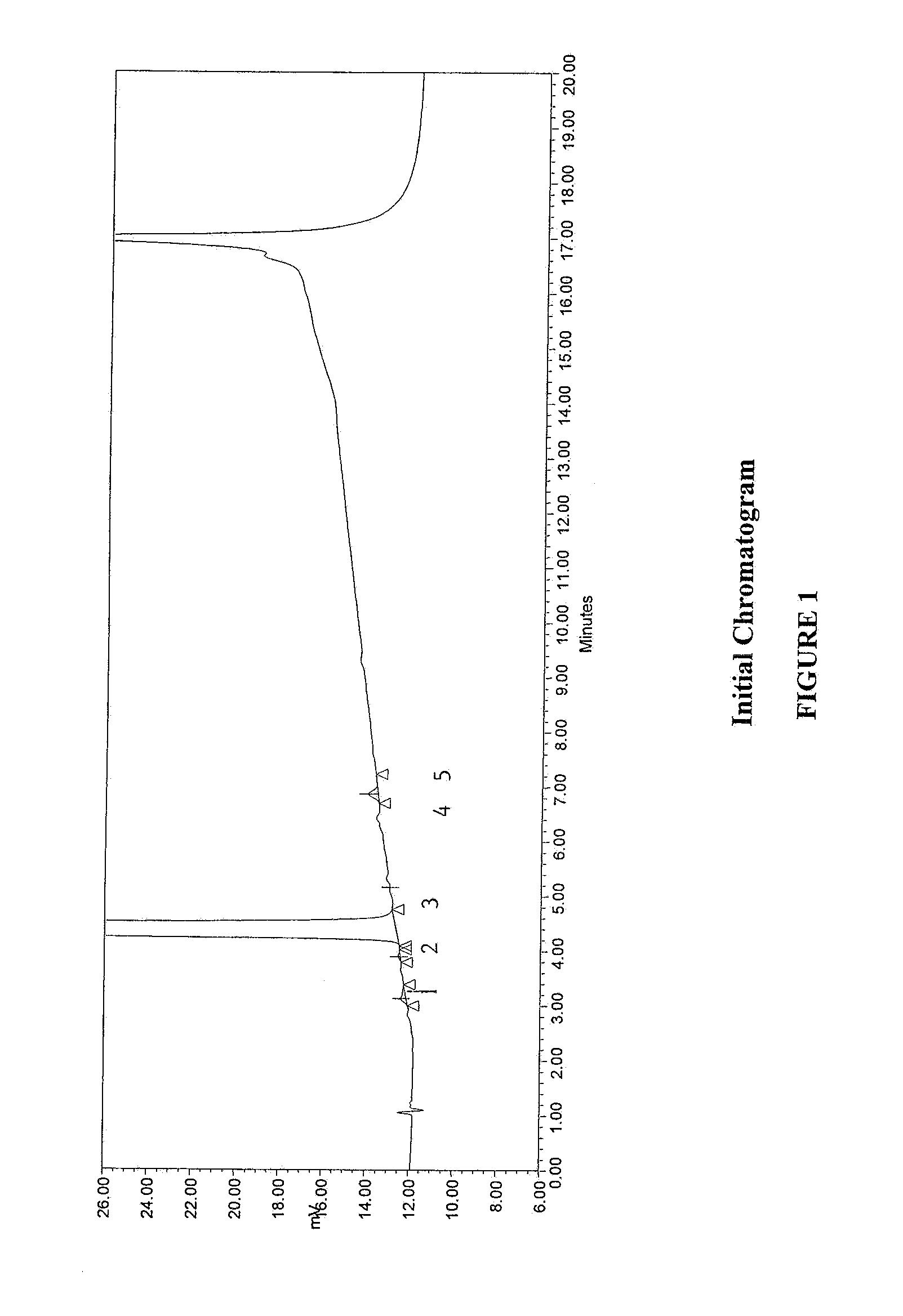
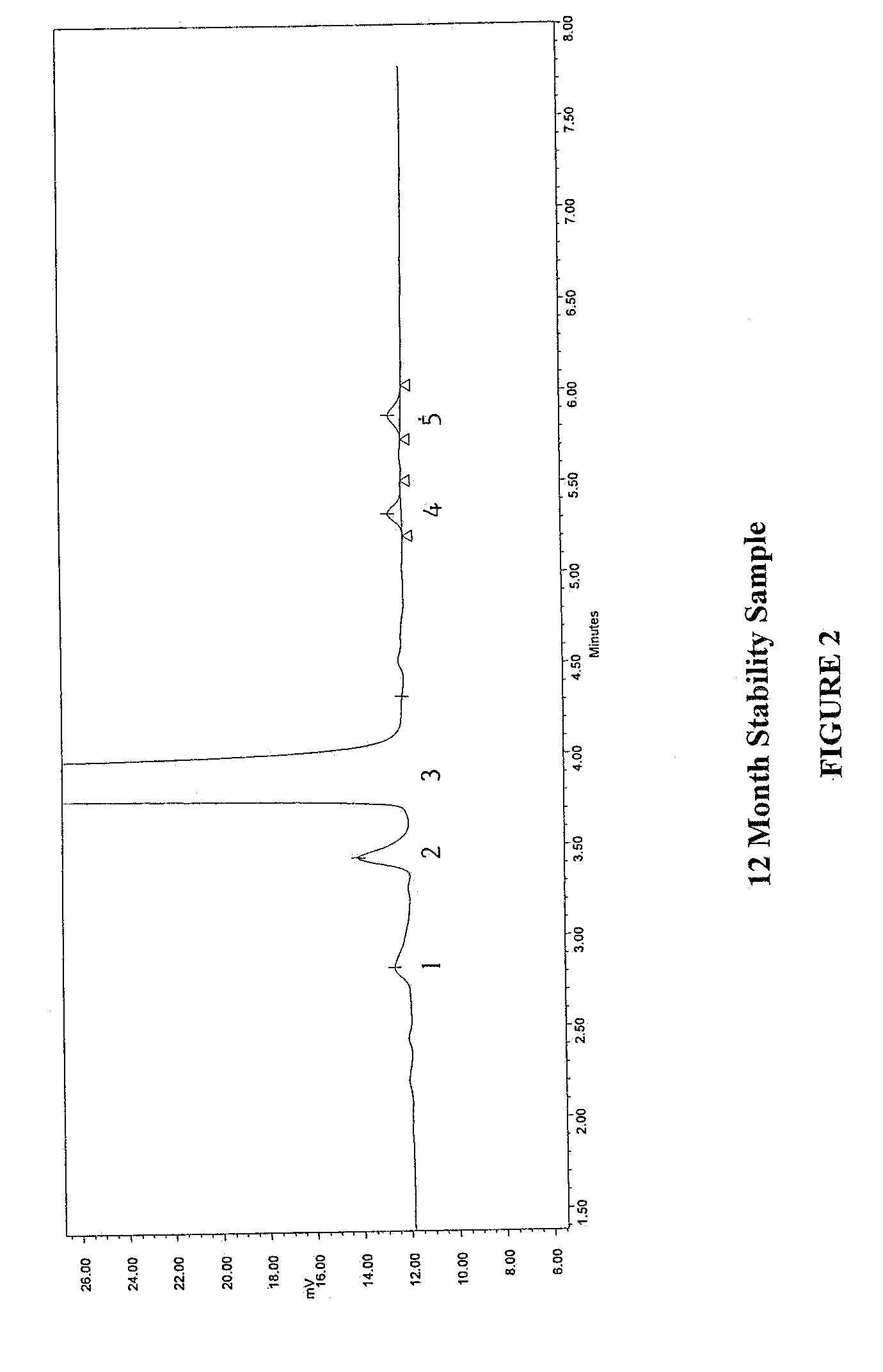
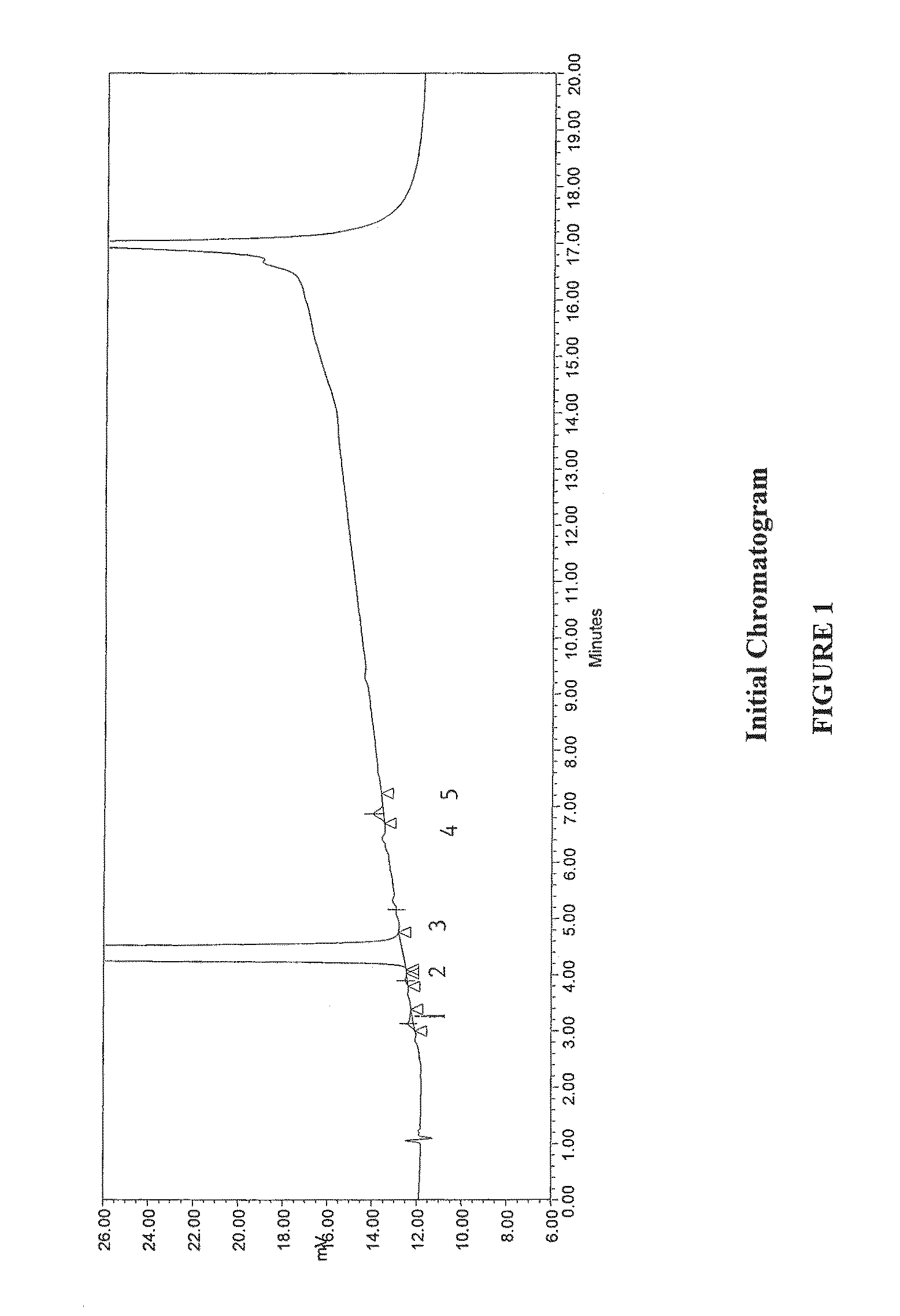
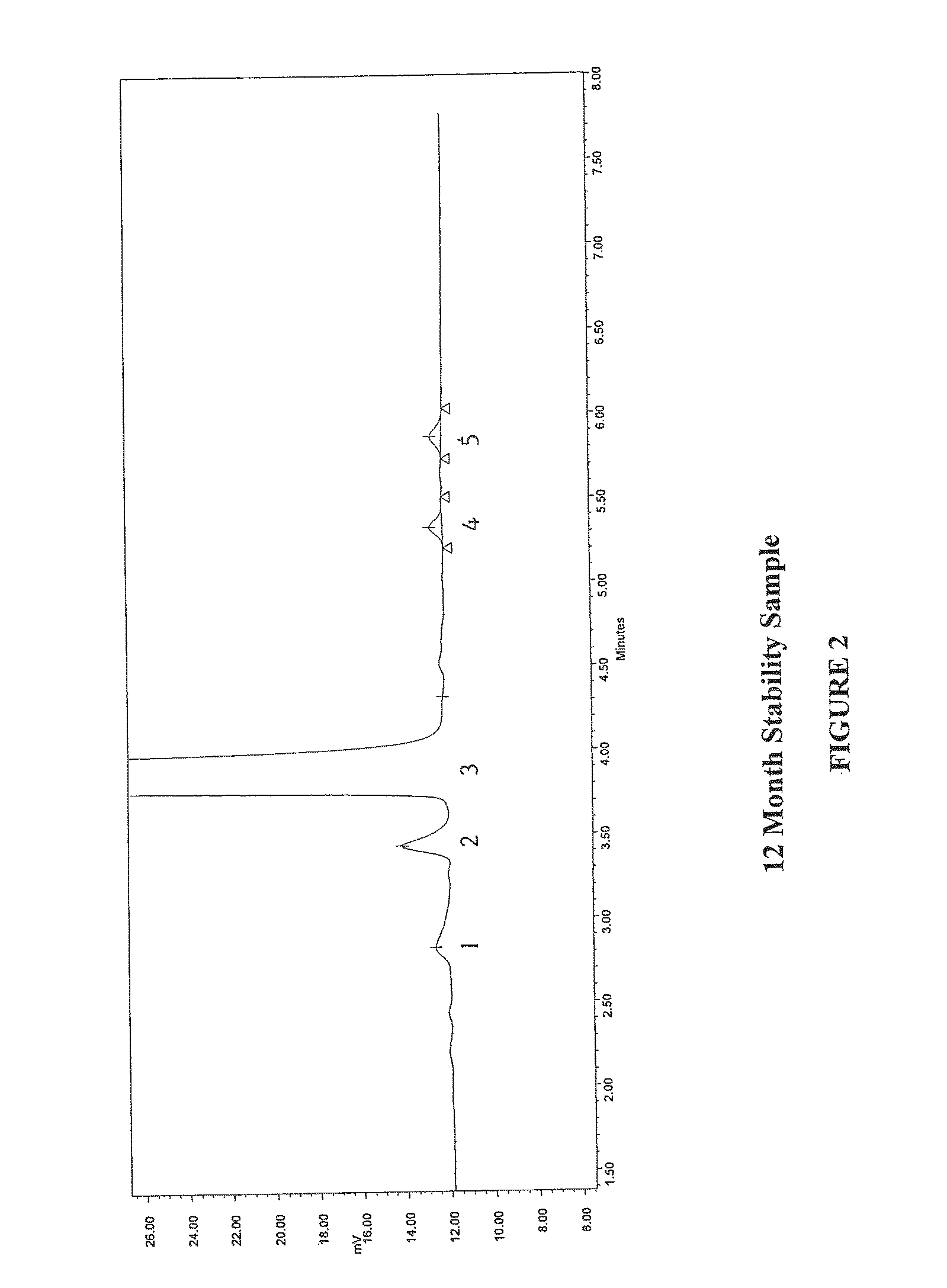
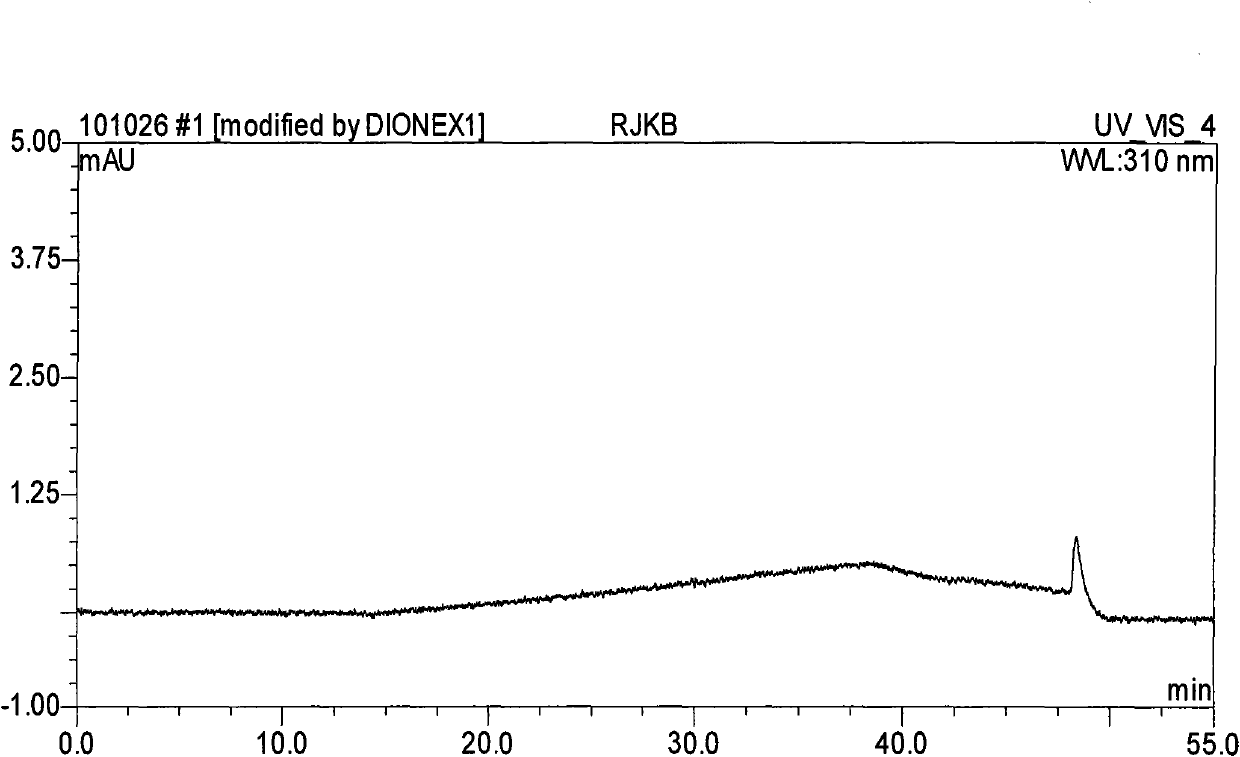
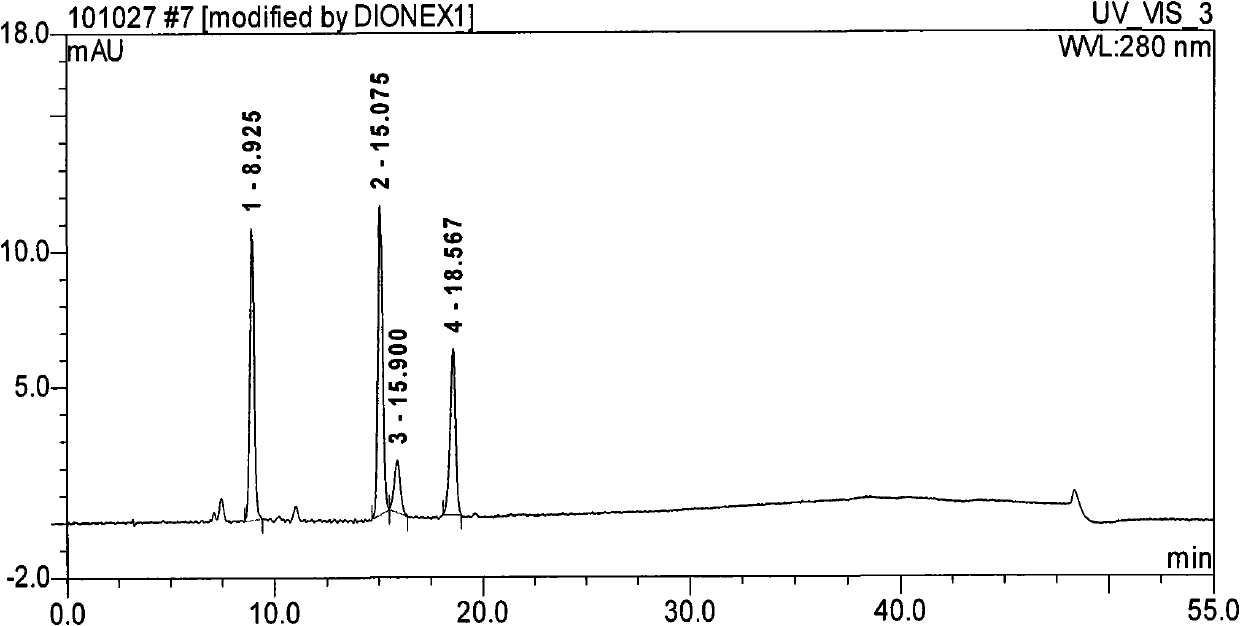
Method for separating and determining methyhaaltrexone bromide and impurities thereof with liquid chromatography
ActiveCN102565203AGood symmetryHigh column efficiencyComponent separationOrganic solventTrifluoroacetic acid
The invention provides a method for separating and determining methyhaaltrexone bromide and impurities and contents thereof with liquid chromatography. The method comprises the following steps: (a) adopting a chromatographic column using alkyl-silicane bonded silica gels as packing to be a separation column; (b) using a trifluoroacetic acid buffer solution as a flowing phase A, and using an organic solvent as a flowing phase B; and (c) and adopting gradient elution for the flowing phases, and separating and determining the impurities and contents of methyhaaltrexone bromide and a preparation thereof. According to the method, methyhaaltrexone and other known intermediate impurities as well as unknown impurities can be effectively separated and determined. The method has strong specificity, high accuracy and convenience for operation.
Owner:CHONGQING PHARMA RES INST
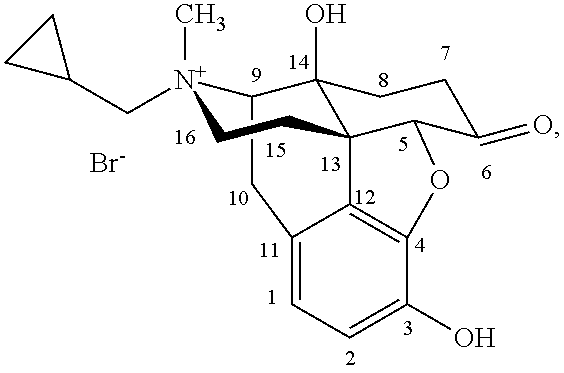
Morphinan derivatives and preparation methods thereof
InactiveUS8563721B2Simple processDifficult to purifySilicon organic compoundsMorphinanMorphinan derivatives
Owner:CHONGQING PHARMA RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com