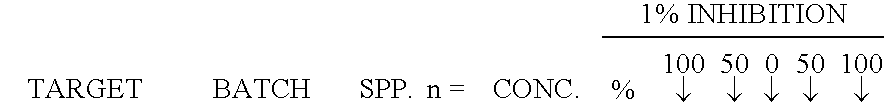Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "OPIATE ANTAGONISTS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
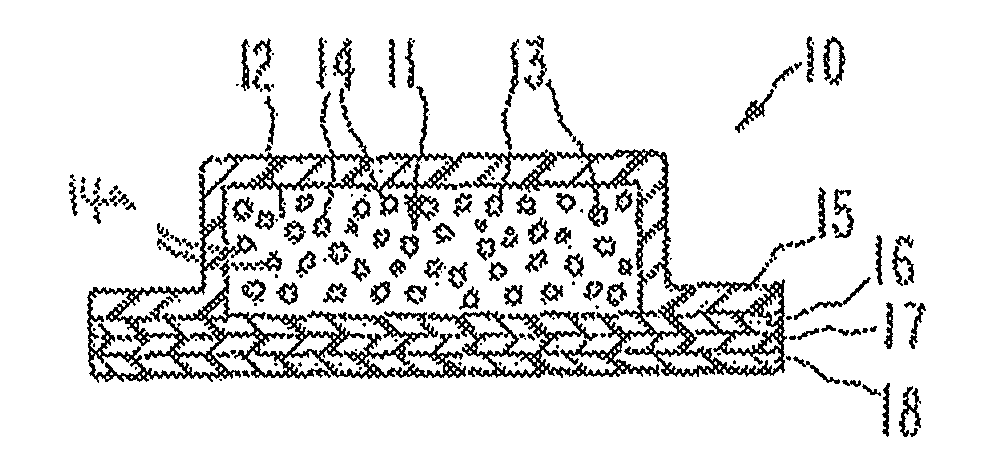
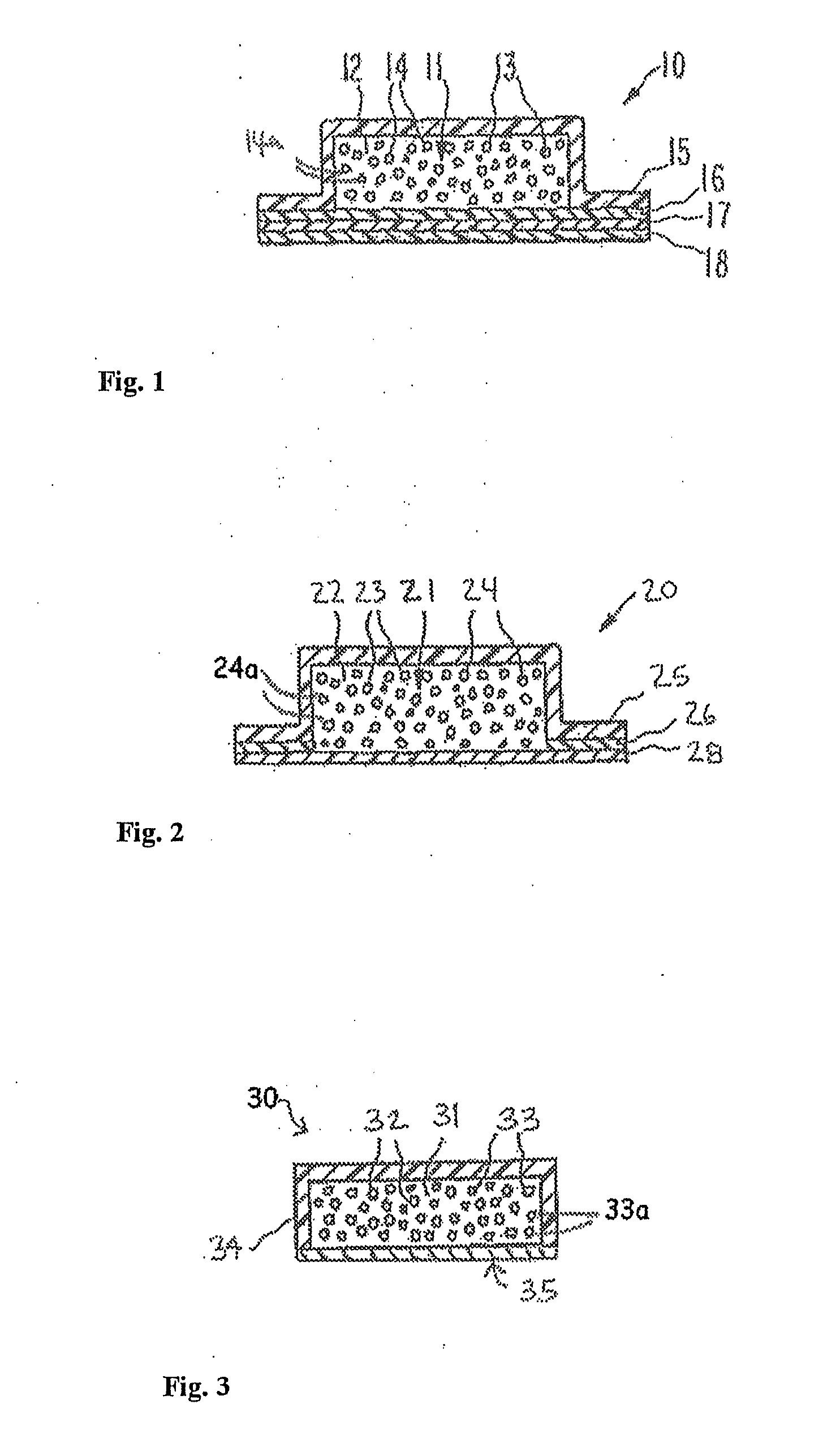
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
Smoking cessation treatments using naltrexone and related compounds
InactiveUS6541478B1Reduce weight gainPrevent relapseBiocideNervous disorderOpioid antagonistAntianxiety Agent
Nicotine dependency is treated by administration of an opioid antagonist. In some embodiments, rapid or ultra rapid detoxification techniques include using a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone or naltrexone or a mixture of any one of these, and either clonidine or related compounds either while awake, or while under sedation or anesthesia, followed by continued administration of an effective amount of an opioid antagonist with or without agents that enhance nicotine dependency treatment. Persons are also treated for nicotine dependency with more gradual detoxification methods using administration of a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone, naltrexone, or a mixture of any of these, and an effective amount of agents used to treat nicotine withdrawal including nicotine, such as that delivered by a nicotine patch, nicotine chewing gum, nicotine inhaler or other methods for delivering nicotine, antidepressants and antianxiety agents, and / or clonidine and related compounds. Administration of an effective amount of an opioid antagonist to prevent relapse, attenuate craving, and reduce weight gain during and after treatment for nicotine dependency is continued in some embodiments.
Owner:YALE UNIV
Methods and materials for the treatment of pain comprising opioid antagonists
InactiveUS20050038062A1Enhance neuropathic pain-alleviating potencyEnhancing the potency of opioid agonistsBiocideNervous disorderOpioid AgonistOpioid antagonist
Methods and compositions for treating subjects with pain, including neuropathic pain, using opioid antagonists or combinations of opioid antagonists and opioid agonists, including, for example, wherein the amount of an opioid antagonist enhances the neuropathic pain-alleviating potency of an opioid agonist.
Owner:PAIN THERAPEUTICS INC
Transdermal dosage form comprising an active agent and a salt and a free-base form of an adverse agent
InactiveUS20080020028A1Sufficient amountInhibition effectBiocideNervous disorderOpioid antagonistPreventing pain
This invention relates to a tamper-resistant transdermal dosage form comprising an active agent, such as an opioid, or a pharmaceutically acceptable salt thereof, a free base of an adverse agent, such as an opioid antagonist, and a pharmaceutically acceptable salt of an adverse agent, such as an opioid antagonist. The transdermal dosage form allows an effective amount of the active agent, or a pharmaceutically acceptable salt thereof, to be transdermally administered to an animal. The invention further relates to methods for treating or preventing pain in an animal comprising contacting the skin of an animal in need thereof with the transdermal dosage form of the invention for an amount of time sufficient to treat or prevent pain.
Owner:PURDUE PHARMA LP
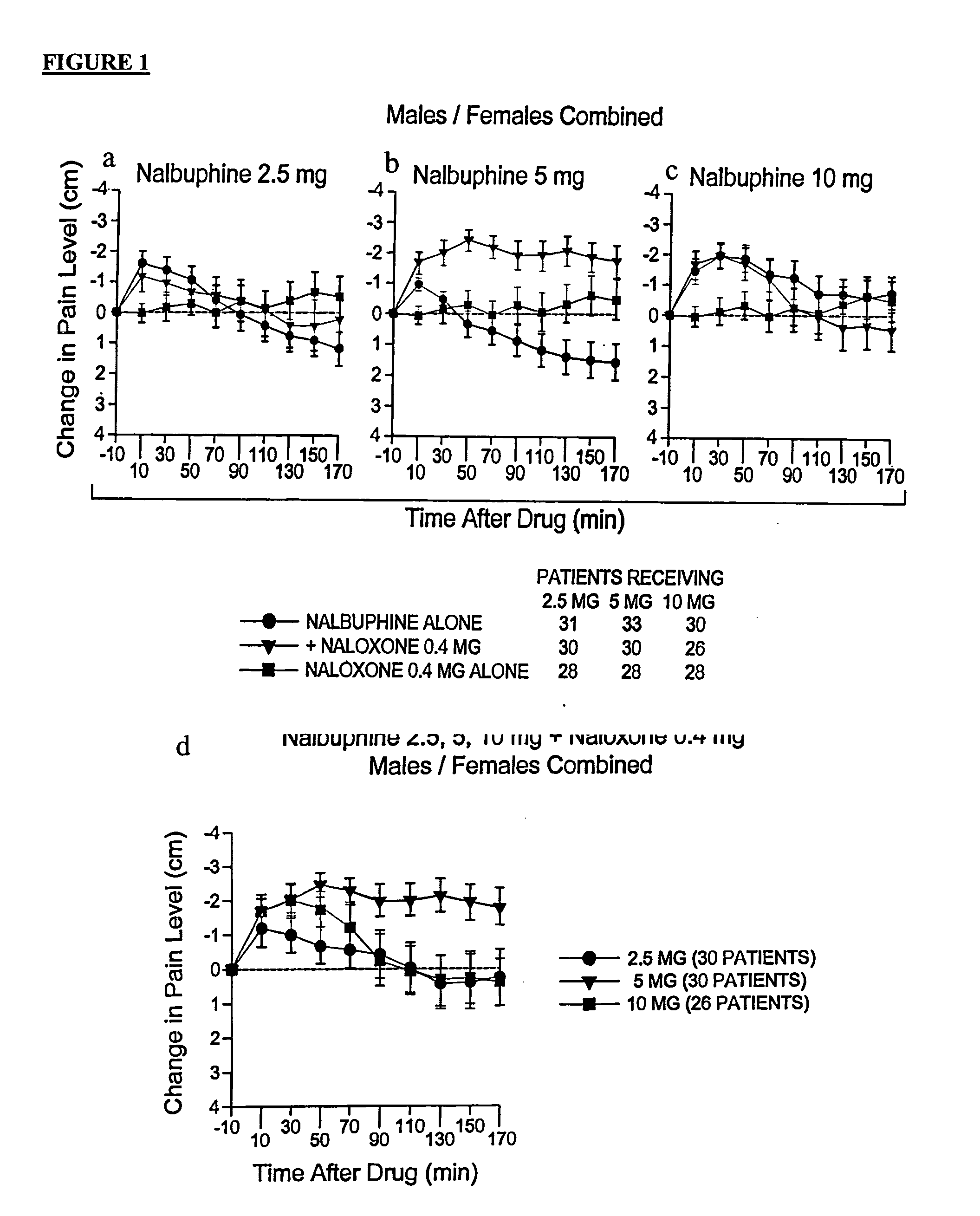
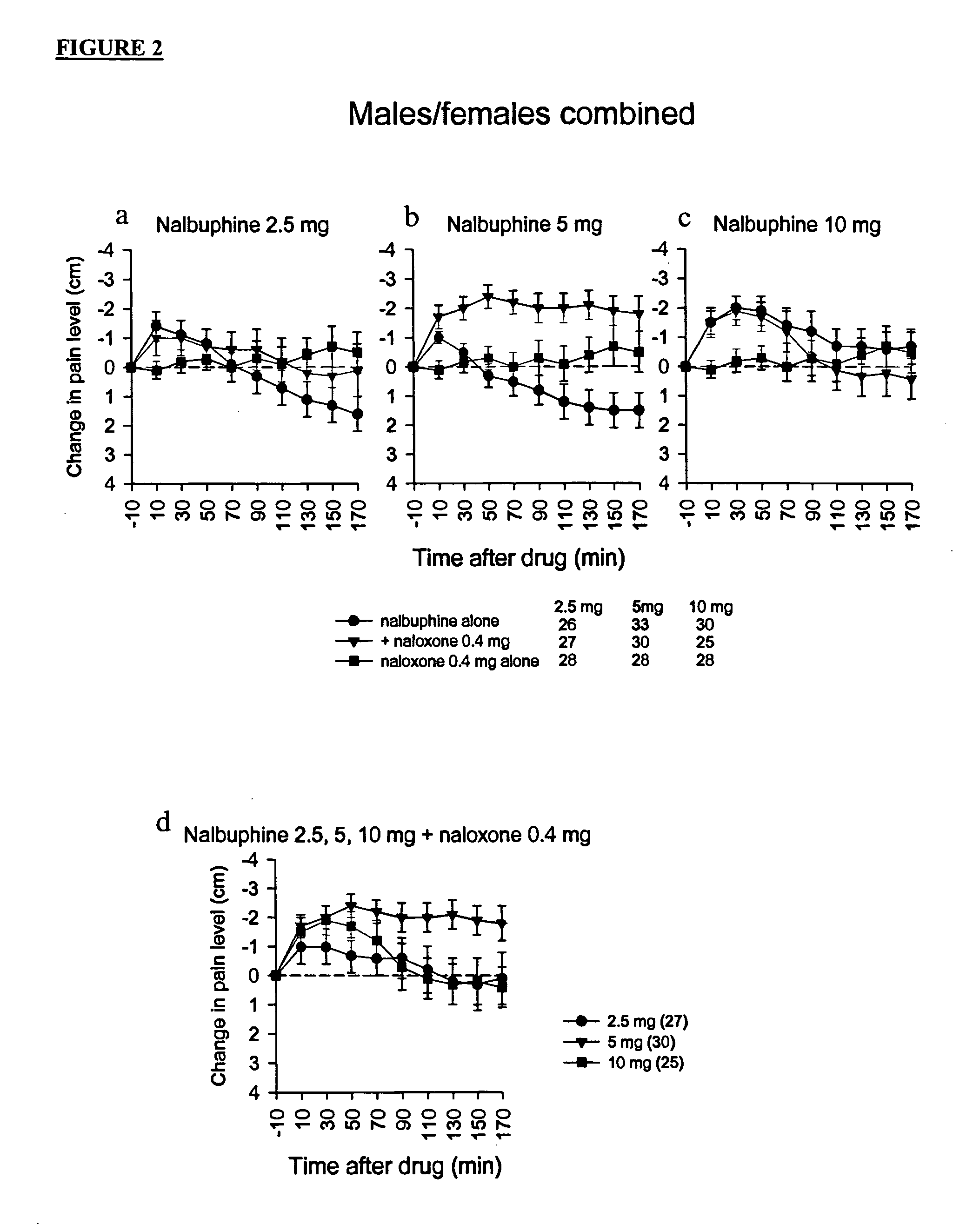
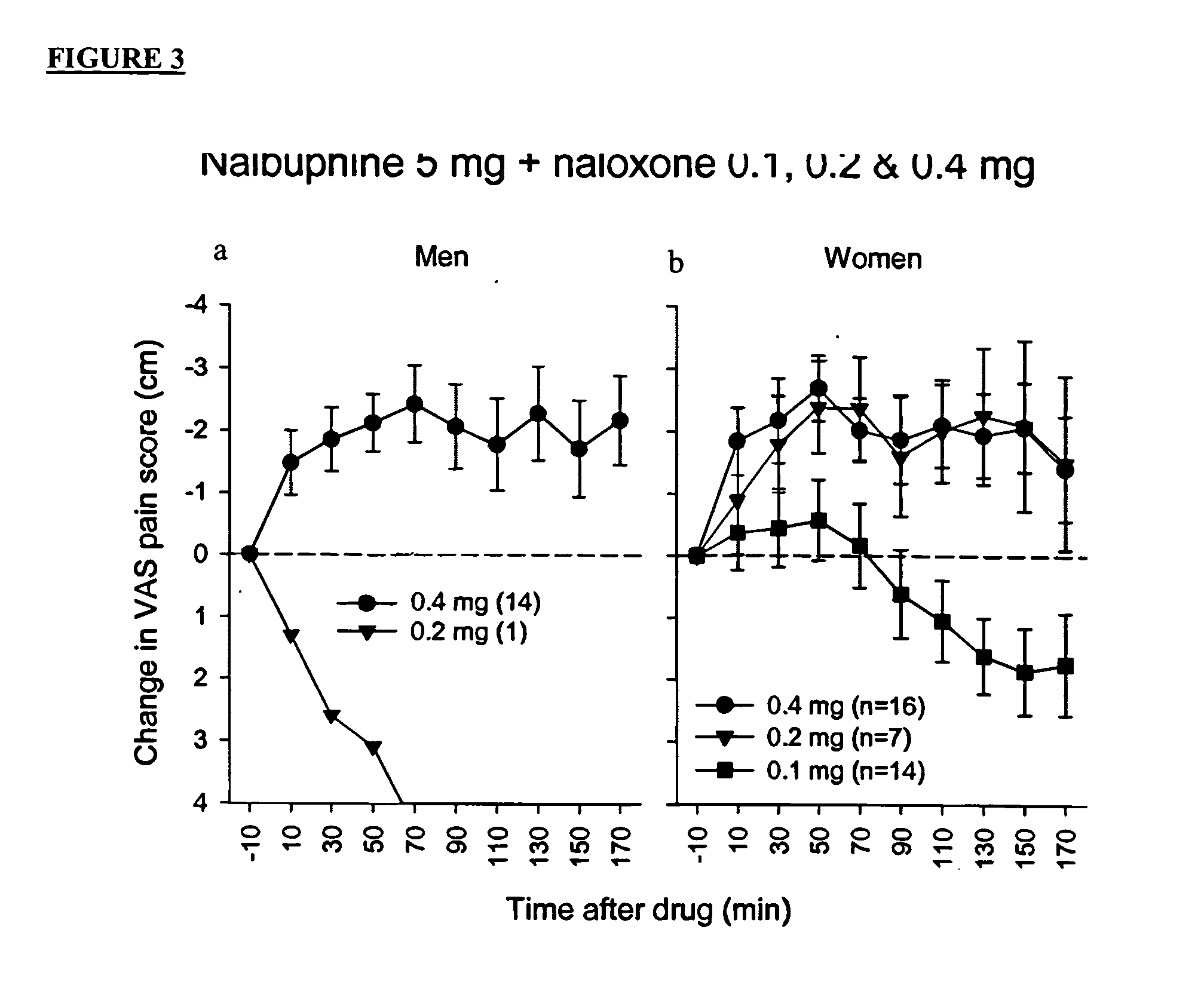
Treatment of pain with combinations of nalbuphine and other kappa-opioid receptor agonists and opioid receptor antagonists
InactiveUS20040180916A1Enhances nalbuphine analgesiaMinimize ToxicityBiocideNervous disorderOpioid antagonistPentazocine
Methods and compositions for treating, managing or ameliorating pain in a subject (preferably, a mammal, and more preferably, a human) comprising administration of a centrally acting (i.e., crosses the blood brain barrier) agonist of a kappa-opioid receptor and a centrally acting opioid antagonist such that the analgesia achieved by this administration is greater than with administration of either the kappa-opioid receptor agonist or the opioid antagonist alone. Preferably the kappa-opioid receptor is nalbuphine or a salt or prodrug of nalbuphine and the opioid antagonist is naloxone or a salt or prodrug of naloxone. Preferred methods of administration include mucosal (e.g. intranasal or pulmonary) and intravenous. Other kappa-opioid receptors include pentazocine and butorphanol.
Owner:RGT UNIV OF CALIFORNIA
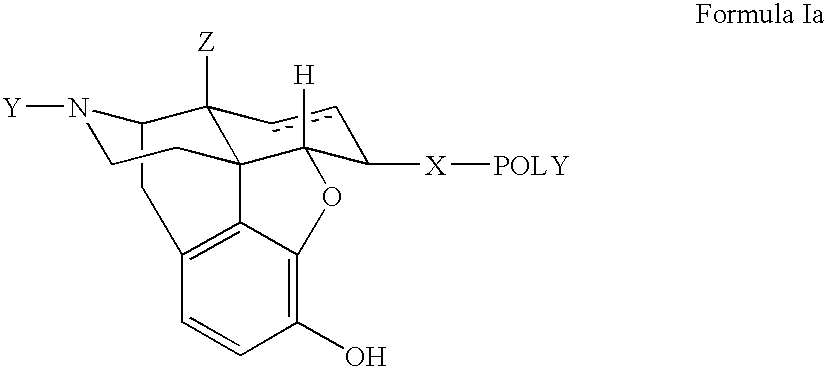
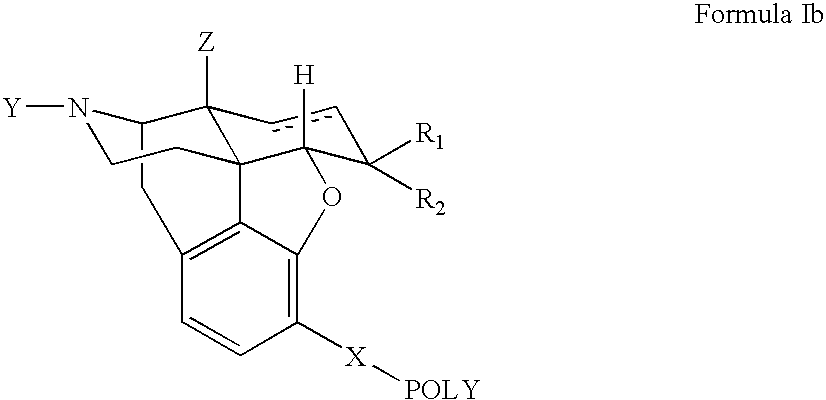
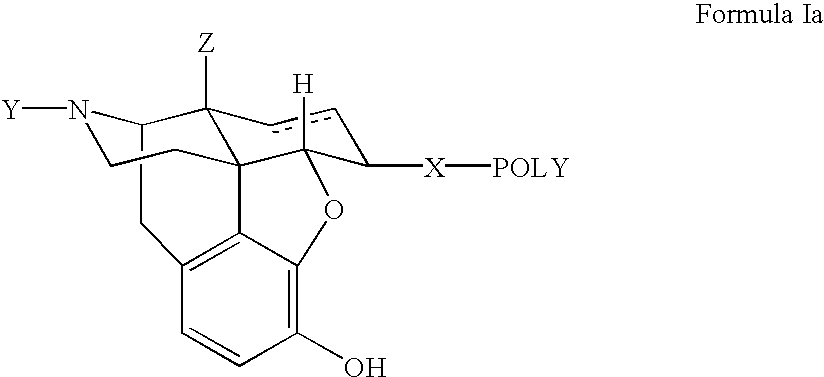
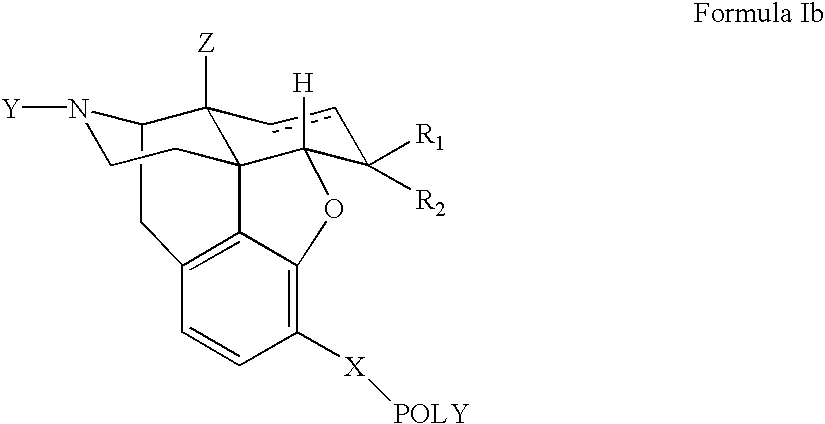
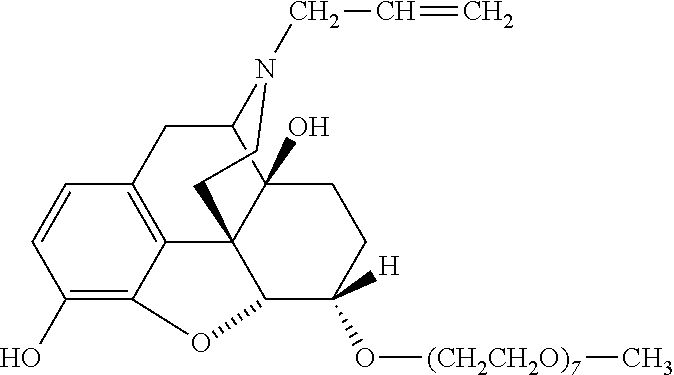
Polymer conjugates of opioid antagonists
The invention provides polymer conjugates of opioid antagonists comprising a polymer, such as poly(ethylene glycol), covalently attached to an opioid antagonist. The linkage between the polymer and the opioid antagonist is preferably hydrolytically stable. The invention also includes a method of treating one or more side effects associated with the use of opioid analgesics, such as constipation, nausea, or pruritus, by administering a polymer conjugate of the invention.
Owner:NEKTAR THERAPEUTICS INC
Polymer conjugates of opioid antagonists
The invention provides polymer conjugates of opioid antagonists comprising a polymer, such as poly(ethylene glycol), covalently attached to an opioid antagonist. The linkage between the polymer and the opioid antagonist is preferably hydrolytically stable. The invention also includes a method of treating one or more side effects associated with the use of opioid analgesics, such as constipation, nausea, or pruritus, by administering a polymer conjugate of the invention.
Owner:NEKTAR THERAPEUTICS INC
Abuse-resistant controlled-release opioid dosage form
InactiveUS20080069881A1High oral : parenteral potency ratioEliminate the effects ofBiocidePowder deliveryOpioid antagonistControl release
Abuse-resistant, controlled release opioid tablets are a combination containing an opioid antagonist such as naloxone at a level above that needed to suppress the euphoric effect of the opioid, if the combination were crushed to break the controlled release properties causing the opioid and opioid antagonist to be released as an immediate release product as a single dose. The controlled release nature of the tablet prevents the accumulation of orally effective amounts of opioid antagonist when taken normally. The opioid antagonist is contained in a controlled-release matrix and released, over time, with the opioid.
Owner:PURDUE PHARMA LP
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
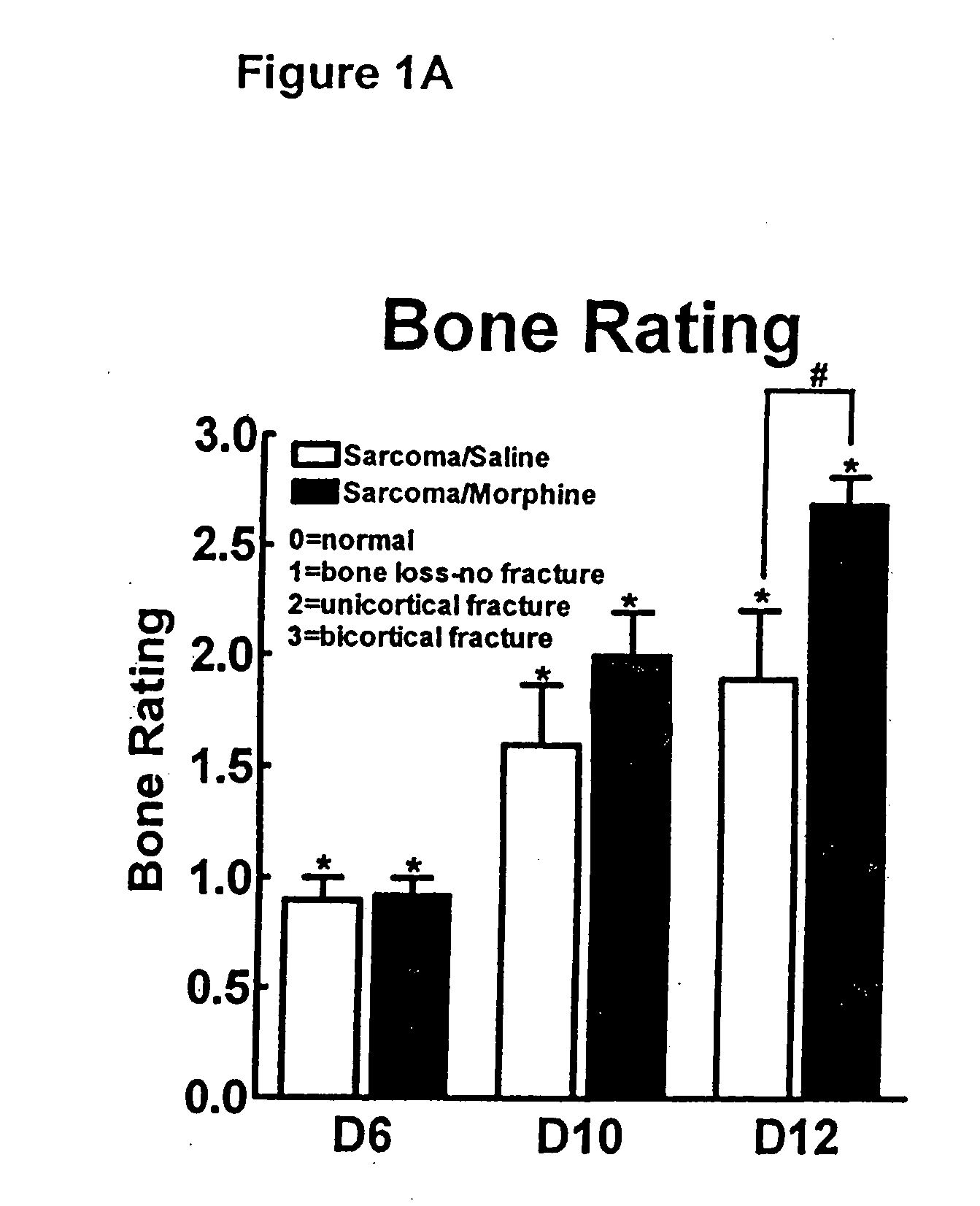
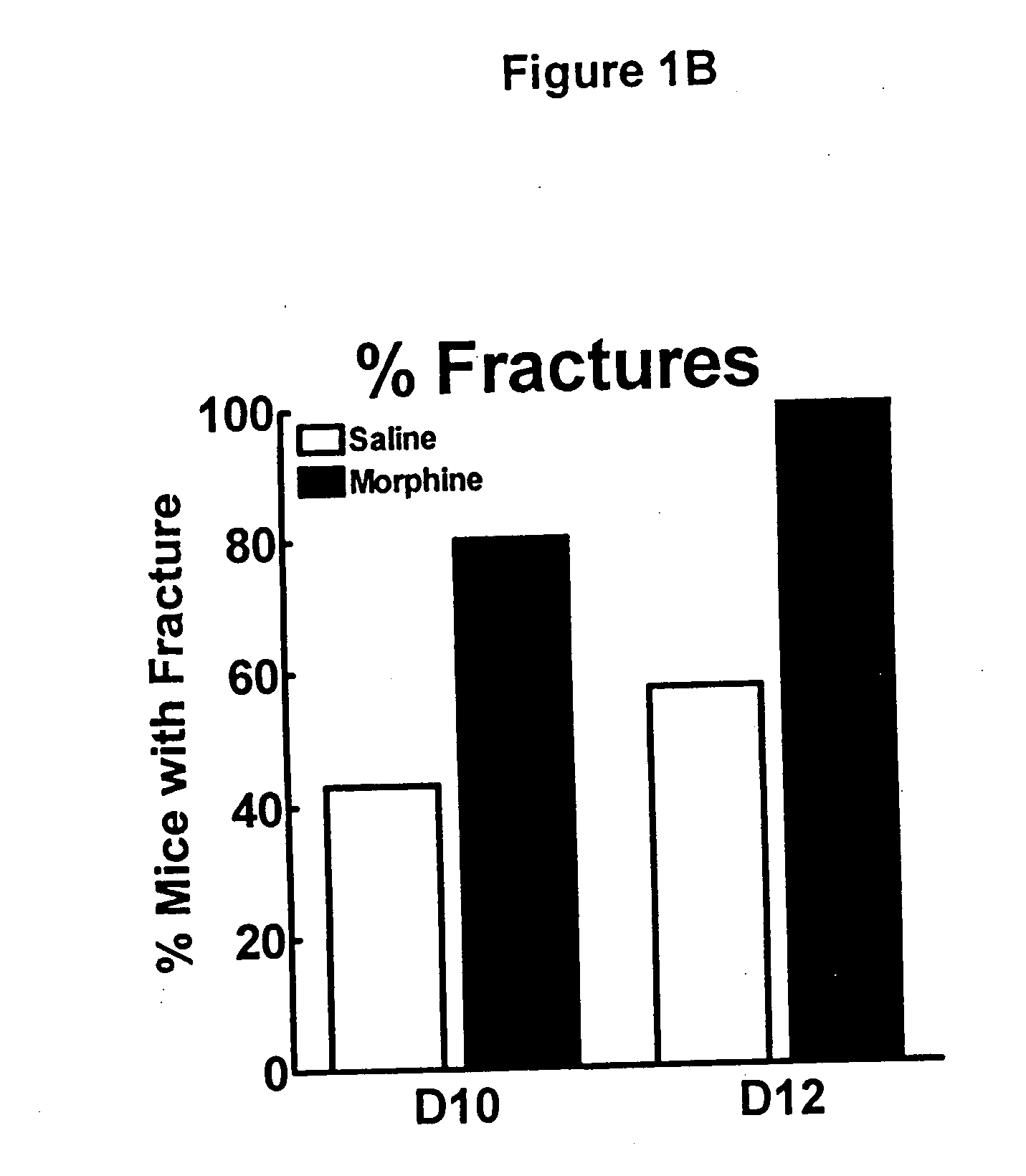
Compositions and methods in the treatment of bone metabolic disorders
InactiveUS20070197573A1Block undesirable peripheral effectAvoid abuseBiocideAnimal repellantsDiseaseOpioid antagonist
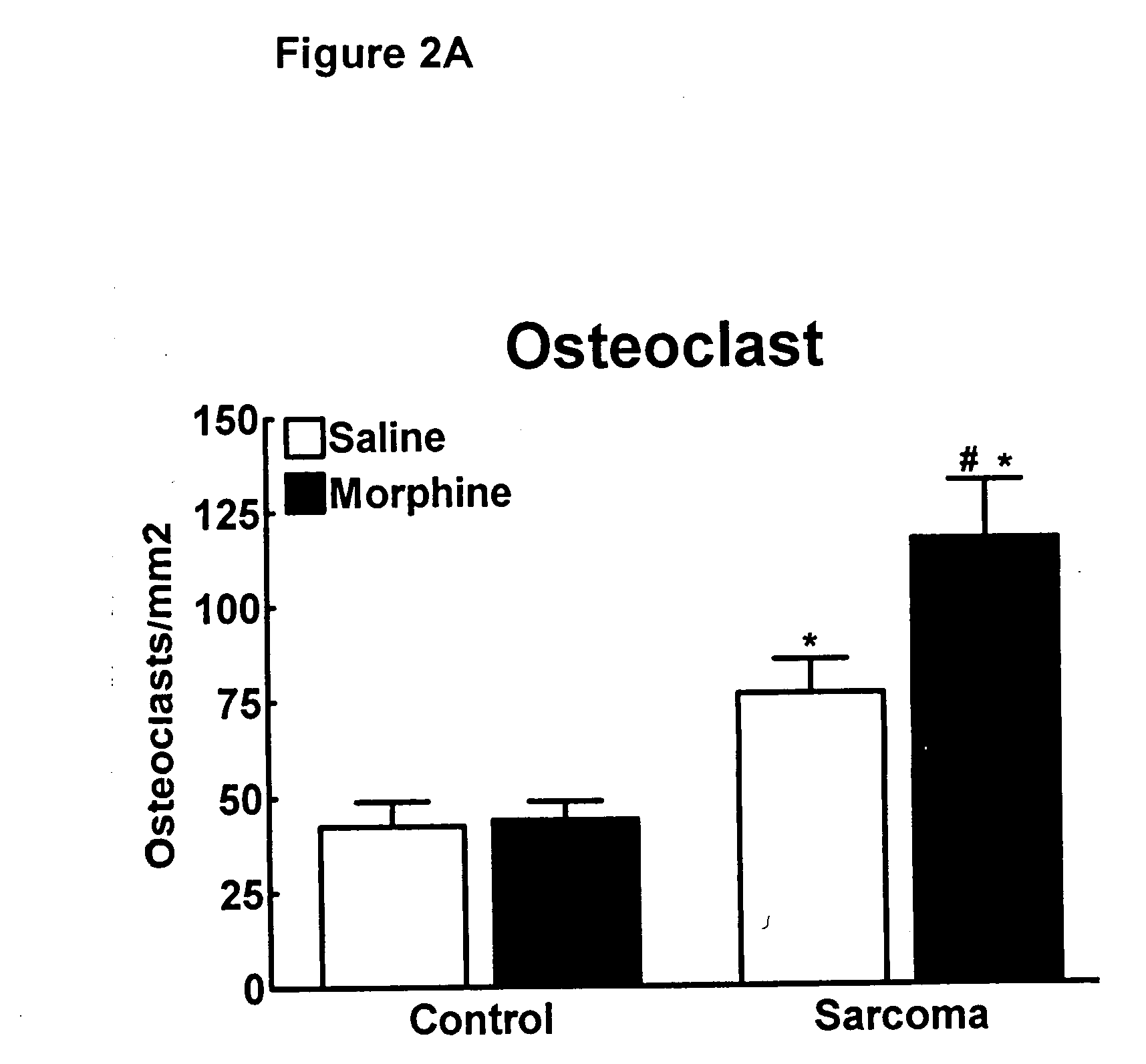
Bone metabolic disorders are treated by administering to an individual a therapeutically effective amount of a peripheral opioid antagonist at one or more of the opioid receptors, including the various naloxone and naltrexone analogs or a pharmaceutically acceptable salt thereof. The invention is further embodied in the use of peripheral antagonists of the opioid receptors, such as the use of naltrexone and naloxone analogs, which can be opioid antagonist with peripheral selectivity at the μ opioid receptor, for the treatment of bone loss, osteoporosis, osteopenia and other bone disorders in individuals using opioid drugs, including patients using opioids for analgesia and in opioid drug-dependent individuals
Owner:AIKO BIOTECH
Formulations and methods for preventing and treating substance abuse and addiction
InactiveUS20060204601A1Maximizing preventionMaximizing treatmentBiocideAnimal repellantsSubstance abuserNR1 NMDA receptor
The invention relates to formulations and methods for preventing and / or treating substance abuse, addiction, withdrawal symptoms, and anticipation associated with substance abuse. In particular the invention relates to formulations comprising at least one processed Morinda citrifolia L. product, and methods comprising the administration of at least one processed Morinda citrifolia L product to prevent and / or treat substance abuse, addiction, withdrawal symptoms, and anticipation associated with substance abuse in living organisms. Embodiments of the invention further comprise Morinda citrifolia based nutraceuticals and administration thereof to affect regulation of dopamine levels, inhibition of opioid receptors, inhibition of mu opioid receptors, inhibition of kappa opioid receptors, inhibition of delta opioid receptors, acting as NMDA antagonist, inhibition of sensitization of NMDA receptors, and / or acting as opiate antagonists.
Owner:TAHITIAN NONI INT INC
Analgetic dosage forms that are resistant to parenteral and inhalation dosing and have reduced side effects
The invention provides a novel solid pharmaceutical dosage form which includes an opiate, an opiate antagonist admixed with the analgetic (opiate agonist) and an amount of a hydrocolloid containing excipient which is effective to form a non-injectable slurry when the dosage form is contacted with water. In addition the dosage form contains pure naloxone in enteric coated form which is designed to release in the colon to prevent or relieve constipation. Thus the formulation, because of the enteric coated naloxone and the hydrocolloid excipient(s), has reduced side effects as compared with formulations which do not contain these features.
Owner:GORDON MAXWELL
Method of treating the syndrome of lipodystrophy
Methods of treating a human suffering from the Syndrome of Lipodystrophy or one or more HIV-related abnormalities included therein are provided. One method may include administering, by a pharmaceutically effective mode, a drug composition comprising an opioidergic agent, or alternatively, an opioidergic agent and an insulin secretagogue. The method may also include administering, by a pharmaceutically effective mode, a drug composition comprising an opiate agonist and opiate antagonist, or alternatively, an opiate agonist, opiate antagonist and an insulin secretagogue.
Owner:NEURENDO PHARMA LLC
Medicament delivery device for administration of opioid antagonists including formulations for naloxone
ActiveUS20140052069A1Prevent undesired leachingMedical devicesMedical syringesOpioid antagonistPharmaceutical drug
Medicament delivery devices for administration of opioid antagonists are described herein. In some embodiments, an apparatus includes a housing, a medicament container disposed within the housing and an energy storage member disposed within the housing. The medicament container is filled with a naloxone composition that includes naloxone or salts thereof, a tonicity-adjusting agent, and a pH-adjusting agent, whereby the osmolality of the naloxone composition ranges from about 250-350 mOsm and the pH ranges from about 3-5. The energy storage member is configured to produce a force to deliver the naloxone composition.
Owner:KALEO INC
Dosage Forms and Co-Administration of Opioid Agonist and Opioid Antagonist
The present invention provides compositions comprising an opioid agonist and a water-soluble, non-peptidic polymer-opioid antagonist conjugate. Among other things, dosage forms and methods of administering the dosage forms are also provided.
Owner:NEKTAR THERAPEUTICS AL CORP
Accelerated opiate dependence detoxification process
The present invention provides accelerated detoxification methods for the treatment of a substance abuse-related condition in a subject. A method of the present invention may comprise administering to the subject an effective amount of at least one sedative (e.g, clonidine, diazepam, or olanzapine) and a micro-dose of an opioid antagonist (e.g., naltrexone or naloxone) for at least one day; optionally administering a small dose of an opiate, and administering to the subject a detoxifying amount of a second opioid antagonist (e.g., naloxone); and may further comprise administering to the subject a third opioid antagonist (such as, naltrexone) for an extended period of time (e.g., 12 months).
Owner:COLEMAN PETER R
Peripheral Opioid Agonists and Peripheral Opioid Antagonists
The present disclosure provides compositions, and their methods of use, where the compositions comprise a ketone-modified opioid drug, wherein the drug comprises a ketone-modified opioid and a substituent on the opioid that mediates retention of the drug in the peripheral nervous system as opposed to the central nervous system following ingestion by a subject.
Owner:SIGNATURE THERAPEUTICS
Oral administration of peripherally-acting opioid antagonists
InactiveUS20110160239A1Inhibition becomes largerAvoid dysfunctionBiocideNervous disorderOpioid antagonistSide effect
Owner:NEKTAR THERAPEUTICS INC
Method of treating the syndrome of coronary heart disease risk factors in humans
The invention provides an improved method of treating a human suffering from one or more conditions included within the Coronary Heart Disease Risk Factor (CHDRF) syndrome. The method includes administering, by a pharmaceutically effective mode, a drug composition having an opioidergic agent including an opiate antagonist, opiate having μ-agonist activity or combination thereof, and an insulin secretagogue.
Owner:NEURENDO PHARMA LLC
Abuse deterrent opiod/opiod-antagonist transdermal patch
Owner:BUZZZ PHARMA
Processes for the preparation of peripheral opioid antagonist compounds and intermediates thereto
Novel processes for the preparation of peripheral opioid antagonist compounds and intermediates thereto. The compounds prepared by the present processes may be useful, for example, as antagonists to the mu, kappa and delta opioid receptors, and thereby may be useful in the treatment of gastrointestinal motility disorders, and in preventing peripheral opiate induced side effects. The present processes may offer improved yields, purity, ease of preparation and / or isolation of intermediates and final product, and more industrially useful reaction conditions and workability.
Owner:MERCK SHARP & DOHME LLC
Opioid agonist formulations with releasable and sequestered antagonist
Disclosed are oral dosage forms, comprising (i) a therapeutically effective amount of an opioid agonist; (ii) an opioid antagonist in releasable form; and (iii) a sequestered opioid antagonist which is not released when the dosage form is administered intact, and methods thereof.
Owner:PURDUE PHARMA LP
Method of treating the syndrome of coronary heart disease risk factors in humans
The invention provides an improved method of treating a human suffering from one or more conditions included within the Coronary Heart Disease Risk Factor (CHDRF) syndrome. The method includes administering, by a pharmaceutically effective mode, a drug composition having an opioidergic agent including an opiate antagonist, opiate having μ-agonist activity or combination thereof, and an insulin secretagogue.
Owner:CPD LLC
Treatment of alcohol abuse and alcoholism using modulators of neurosteroid binding sites on gabaa receptors
The present invention provides methods for reducing alcohol drinking behavior in humans and treating acute alcohol poisoning through the use of neutral or negative modulating agents of the neurosteroid sites on GABAA receptors. These agents avoid the unwanted side-effects of opiate antagonists and displays specificity toward the neurosteroid binding sites on GABAA receptors.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Method for rapid detoxification of addiction
InactiveUS20050101621A1Increase chanceUltra-rapid detoxificationBiocideAnimal repellantsOpiateWhole body
A process for general anesthesia assisted opiate detoxification comprises administering an opiod antagonist at at least a rate of 0.6 mg / kg / hr to an addicted patient.
Owner:DETOX SOLUTIONS
Processes for the preparation of peripheral opioid antagonist compounds and intermediates thereto
Novel processes for the preparation of peripheral opioid antagonist compounds and intermediates thereto. The compounds prepared by the present processes may be useful, for example, as antagonists to the mu, kappa and delta opioid receptors, and thereby may be useful in the treatment of gastrointestinal motility disorders, and in preventing peripheral opiate induced side effects. The present processes may offer improved yields, chemical or stereochemical purity, ease of preparation and / or isolation of intermediates and final product, and more industrially useful reaction conditions and workability.
Owner:DOLLE ROLAND E +5
Oral administration of peripherally-acting opioid antagonists
Peripherally-acting opioid antagonists can be orally administered to treat the side effects of opioid administration in convenient dosing schedules.
Owner:NEKTAR THERAPEUTICS INC
Use of opioid antagonists
ActiveUS8518962B2Inhibit migrationPrevent proliferationBiocideOrganic chemistryOpioid antagonistLymphatic Spread
Embodiments of the invention provide methods of attenuating, e.g., inhibiting or reducing, cellular proliferation and migration, particularly endothelial cell proliferation and migration, including that associated with angiogenesis, as well as attenuating cancerous tumor growth and metastasis, using opioid antagonists, including, but not limited to, those that are peripherally restricted antagonists.
Owner:UNIVERSITY OF CHICAGO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com