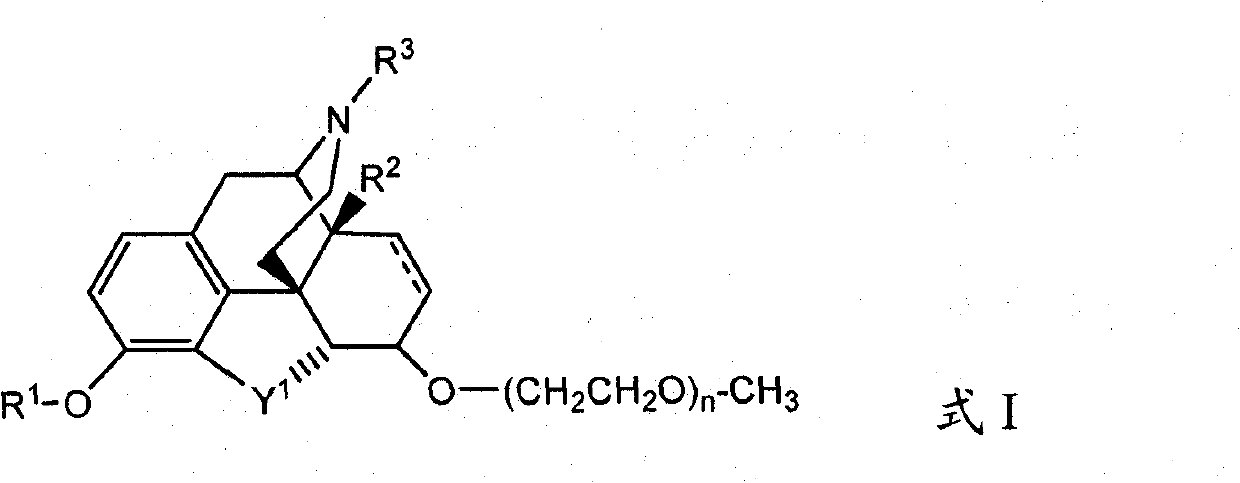
Oral administration of peripherally-acting opioid antagonists
An opioid antagonist, the technology of action, applied in the field of pharmacology and medicine, can solve the problem of inability to eliminate the analgesic effect of opioids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
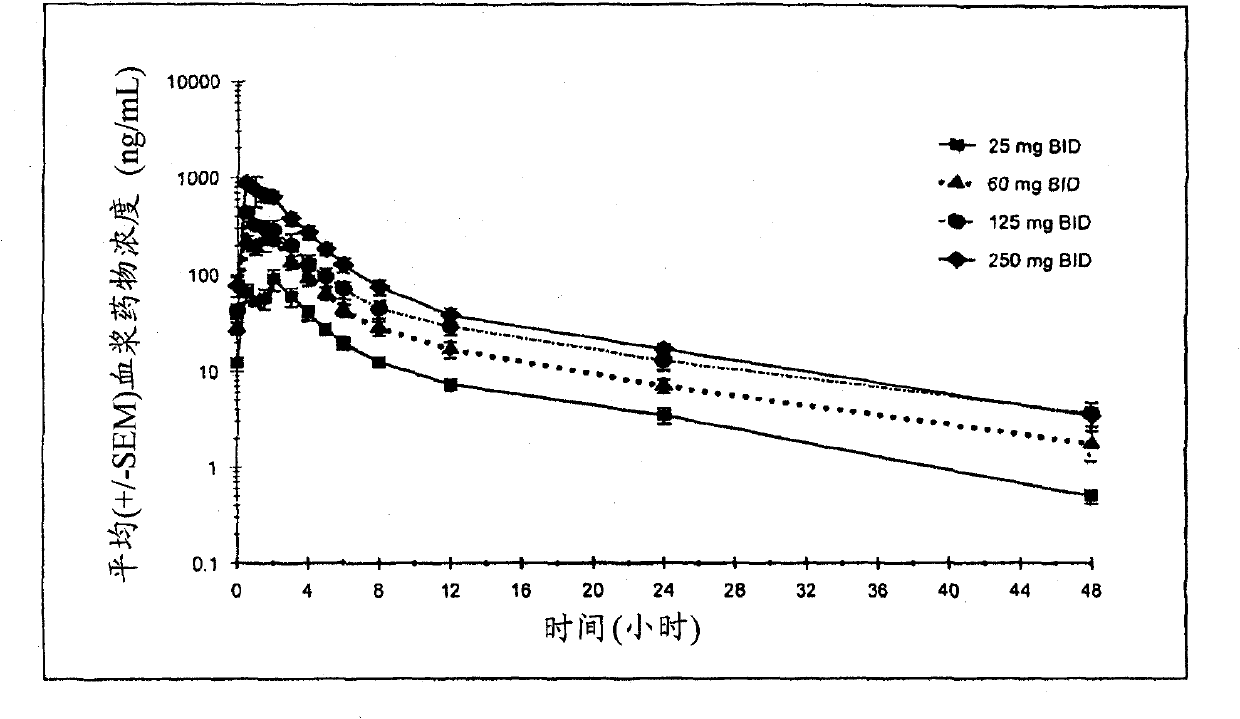
[0082] A double-blind, randomized, placebo-controlled multiple-dose study (double-blind, randomized, placebo-controlled, multiple-dose study) was conducted to evaluate the safety, tolerability and pharmacokinetics of compound I oral doses.
[0083] Thirty-two healthy male and female volunteer subjects were enrolled in this randomized, double-blind, placebo-controlled, multiple-dose dose-escalation study. The main enrollment criteria are: (i) age ≥ 18 and ≤ 65 years old; (ii) body mass index (BMI) ≥ 18 and ≤ 30kg / m 2 ; (iii) non-smokers, no history of drug abuse or alcohol abuse; (iv) normal frequency of bowel movements within the past month; and (v) female subjects must be postmenopausal or surgically menopausal. There were 16 male subjects and 16 female subjects participating in the study. Subjects ranged in age from 25 to 65 years. BMI (weight in kilograms divided by height in meters squared) ranges from 19 to 29.
[0084] The subjects were randomly divided into groups ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com