Novel phosphodi esterase inhibitors
A technology of diclopyridine and dioxide, which is applied in the direction of anti-inflammatory agents, non-central analgesics, active ingredients of heterocyclic compounds, etc., and can solve problems such as adverse reactions and restrictions on the clinical application of theophylline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
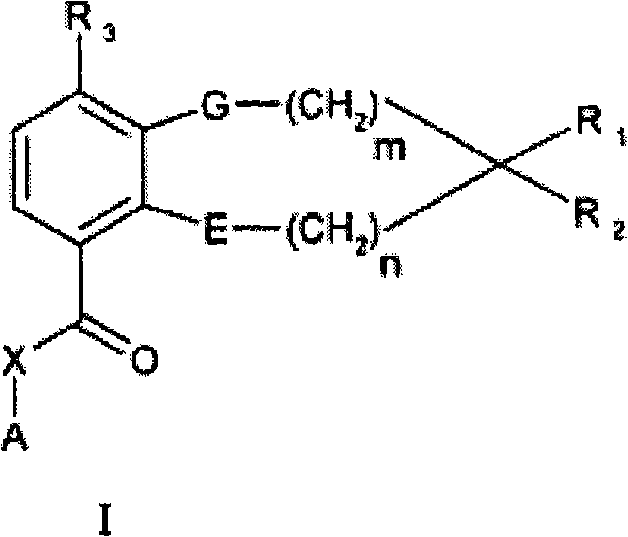
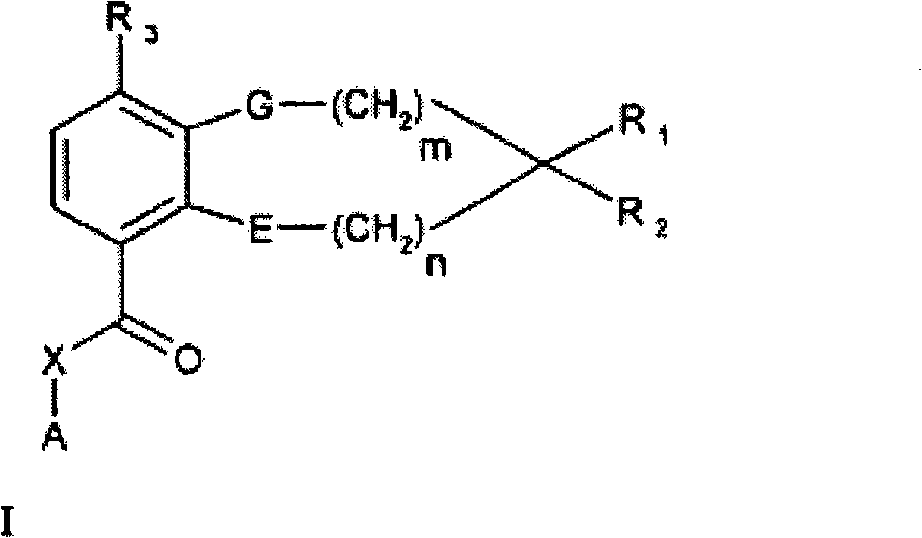
[0047] In one or more embodiments of the invention, both E and G are oxygen.
[0048] In one or more embodiments of the invention, both m and n are one.
[0049] In one or more embodiments of the invention, both m and n are zero.
[0050] In one or more embodiments of the invention, R 1 and R 2 Together with the carbon atom to which it is attached, it contains one or two selected from -O-, -S-, -S(O)-, -S(O 2 )-, -N= and -N(R 5 )-heteroatom heterocyclic ring; one or more carbon atoms in the heterocyclic ring are optionally replaced by one or more identical or different selected from R 4 of substituents.
[0051] In one or more embodiments of the invention, R 1 and R 2 Together with the carbon atom to which it is attached, it contains one or two selected from -O-, -S-, -S(O)-, -S(O 2 )-and-N(R 5 )- heteroatom heterocycloalkyl ring; one or more carbon atoms in the heterocycloalkyl ring are optionally replaced by one or more same or different R 4 of substituents.
[00...
Embodiment 1
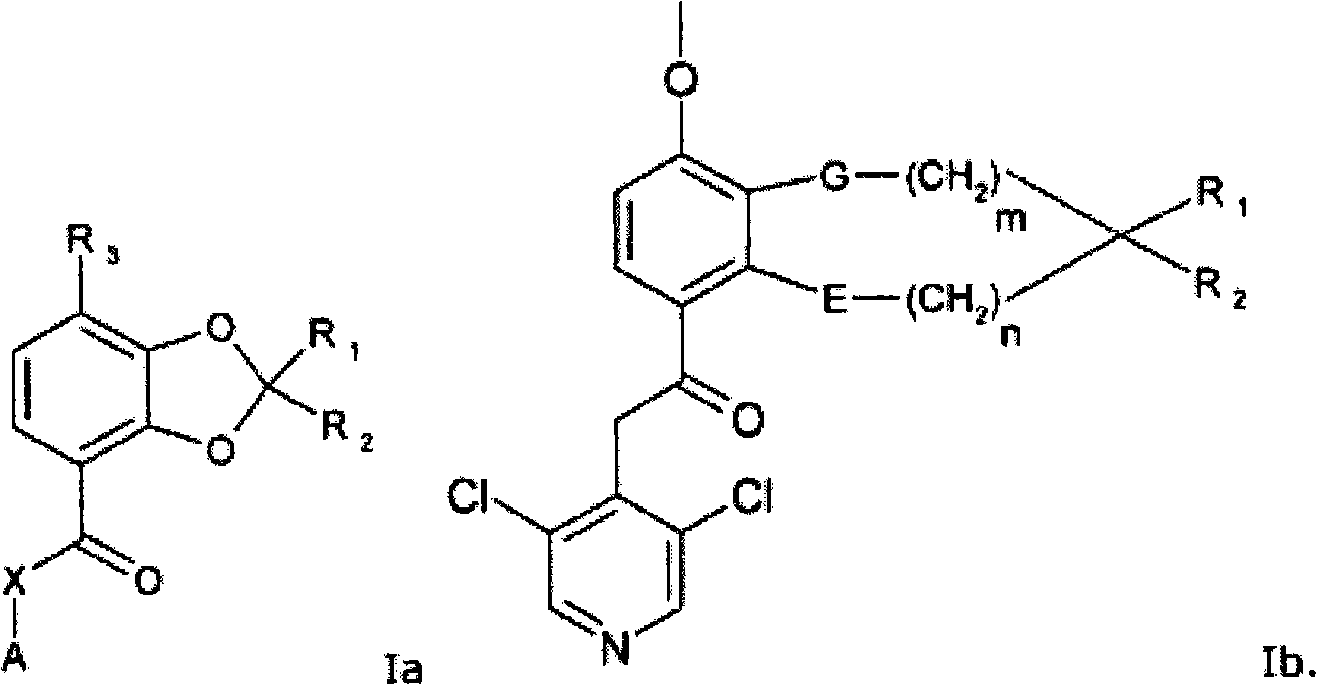
[0194] 2-(3,5-dichloropyridin-4-yl)-1-(7-methoxy-2',3',5',6'-tetrahydro-spiro[1,3-benzodiox Heterocyclopentene-2,4'-(4H)-pyran]-4-yl)ethanone (compound 101)
[0195]
[0196] 7-methoxy-2',3',5',6'-tetrahydro-spiro[1,3-benzodioxole-2,4'-(4H)-pyran]- A solution of methyl 4-carboxylate (1.80 g, 6.42 mmol) and 3,5-dichloro-4-methylpyridine (1.46 g, 8.99 mmol) in tetrahydrofuran (33 mL) was cooled to 0°C. A 1.0 M solution of lithium bis(trimethylsilyl)amide in tetrahydrofuran (19.3 mL, 19.3 mmol) was added and the reaction mixture was allowed to reach room temperature overnight. Add saturated NH 4 Aqueous Cl solution (70 mL). The aqueous phase was extracted with dichloromethane (3 x 100 mL). The combined organic phases were washed with water (50 mL), washed with MgSO 4 Dry and evaporate to dryness under reduced pressure. Standard silica gel column chromatography followed by recrystallization from isopropanol afforded 2-(3,5-dichloropyridin-4-yl)-1-(7-methoxy-2',3',5', 6'-...
Embodiment 2
[0199] N-(3,5-dichloropyridin-4-yl)-7-methoxy-2',3',5',6'-tetrahydro-spiro[1,3-benzodioxolane En-2,4'-(4H)-pyran]-4-carboxamide (compound 102)
[0200]
[0201] Oxalyl chloride (92 μL, 1.1 mmol) and a catalytic amount of N,N-dimethylformamide were added to 7-methoxy-2′,3′,5′,6′-tetrahydro-spiro[1,3 - Benzodioxole-2,4'-(4H)-pyran]-4-carboxylic acid (48 mg, 0.18 mmol) in suspension in dichloromethane (2 mL). After stirring at room temperature for 1 hour, the solvent was removed under reduced pressure and the crude acid chloride was redissolved in tetrahydrofuran (2 mL). A suspension of 3,5-dichloropyridin-4-amine (67 mg, 0.40 mmol) and NaH (60% in mineral oil, 16 mg, 0.40 mmol) in tetrahydrofuran (1 mL) was stirred at room temperature for 3 hours, It was then added dropwise to a solution of the crude acid chloride in tetrahydrofuran at room temperature. After having been stirred overnight at room temperature, the reaction mixture was diluted with diethyl ether (30 mL), and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com