Improved formulations
A preparation and wetting agent technology, which is applied in the field of drug suspension aerosol preparations, can solve problems such as less effective and less robust products, and achieve the effect of reducing the amount of deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
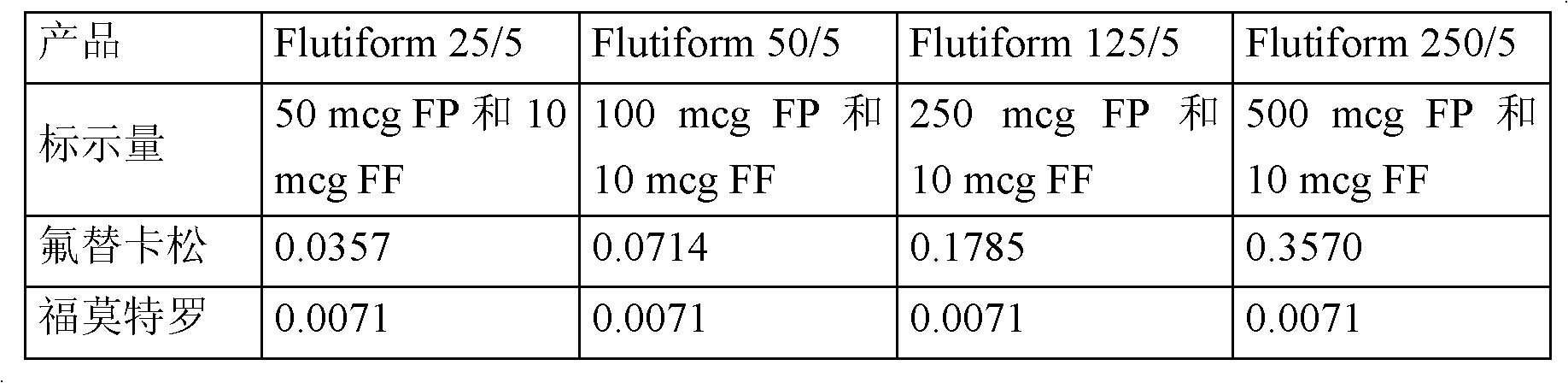
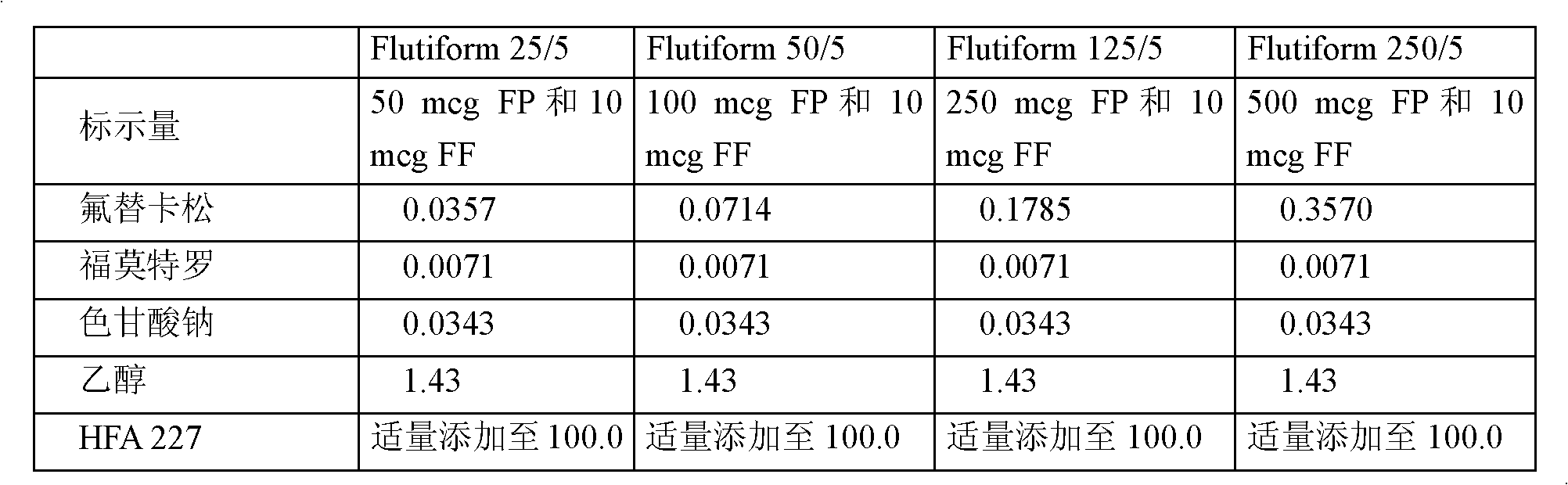
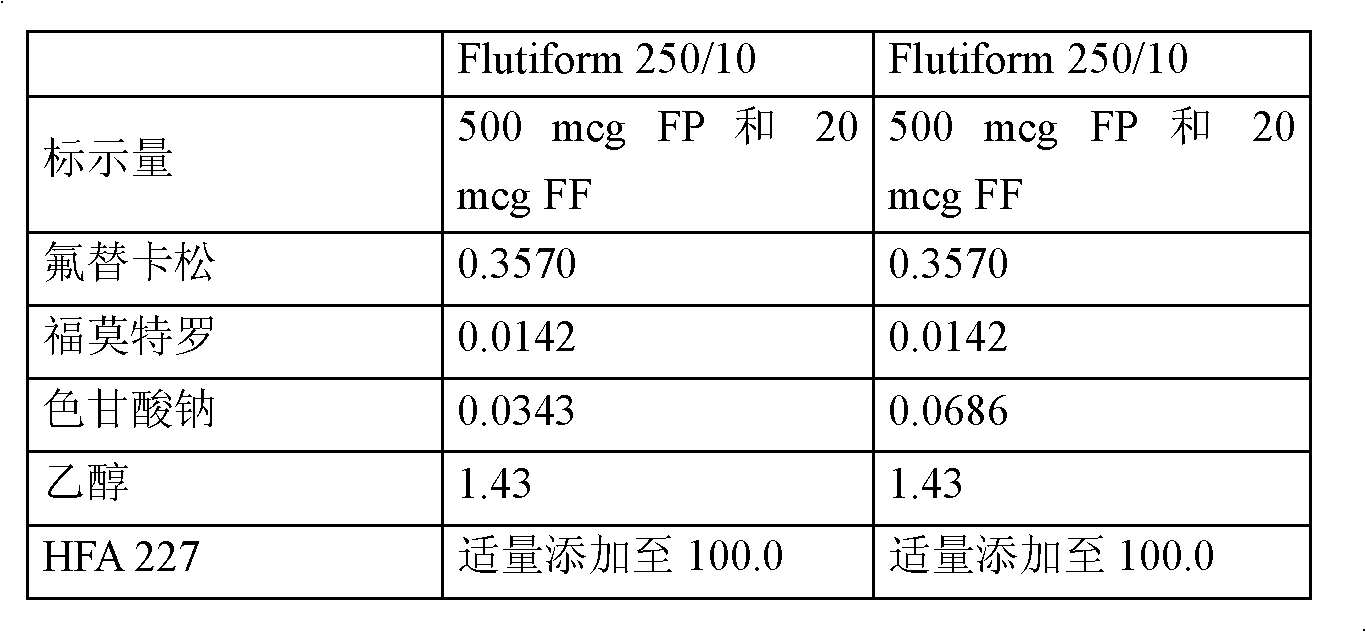
[0094] The following compositions as shown in Table 3 below were prepared.
[0095] Table 3: Pharmaceutical formulation composition
[0096]
[0097] Weigh out an appropriate amount of micronized active material and transfer it to a batch container. Add appropriate amount of micronized sodium cromolyn, (DSCG) and seal the container. A propellant mixture of HFA 227 (Apaflurane) and 1.43% alcohol was prepared in a separate vessel and transferred to the batch vessel. The solid material was dispersed in the liquefied propellant by using a rotor-stator homogenizer at 2900 rpm for 30 minutes. The homogeneous bulk suspension was cooled to 4°C and recirculated between the container and the Pamasol P2001 aerosol filling machine.
[0098] A drug aerosol cartridge with an overflow volume of 14 ml was crimped with a metering valve of 50 mcl using a Pamasol P2005 crimping machine. Aliquots of 11 g of the suspension were filled into crimped cylinders by a P2001 filling machine. The ...
Embodiment 2
[0100] Flutiform 50 / 5 compositions as indicated in Table 2 above were tested against a comparative product identical to the Flutiform 50 / 5 formulation except ethanol was removed from the comparative product.
[0101] MDI cartridges filled with these compositions were tested for analysis of the two drugs in the cartridge, dose consistency throughout the life of the inhaler up to the final labeled amount, aerodynamics of the aerosol drug through the Andersen cascade impactor particle size distribution, and drug deposition on the inner surface of the cartridge and valve (CCS).
[0102] The results are shown in Table 3:
[0103] Table 3: Effect of ethanol on dose uniformity and aerodynamic particle size distribution (APSD). initial results.
[0104]
[0105] The purpose of this experiment was to test the effect of a wetting agent (in this case, dehydrated ethanol) on critical parameters such as dose uniformity and particle size distribution. The results show that the average...
Embodiment 3
[0108] The following batches were prepared using the method described in Example 1:
[0109] Table 14:
[0110]
[0111] As shown in the results shown in Tables 15 and 16 below, the results of the stability investigation up to 12 months demonstrated the excellent product quality and robustness of both formulations.
[0112]
[0113]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com