MSC (mesenchymal stem cell) injection as well as preparation and application thereof
A technology of mesenchymal stem cells and injections, applied in the field of preparation of mesenchymal stem cell injections, can solve the problems of short cell viability maintenance time, inconvenient transportation and storage, etc., to prevent cell aggregation, maintain normal metabolic ability, and composition clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
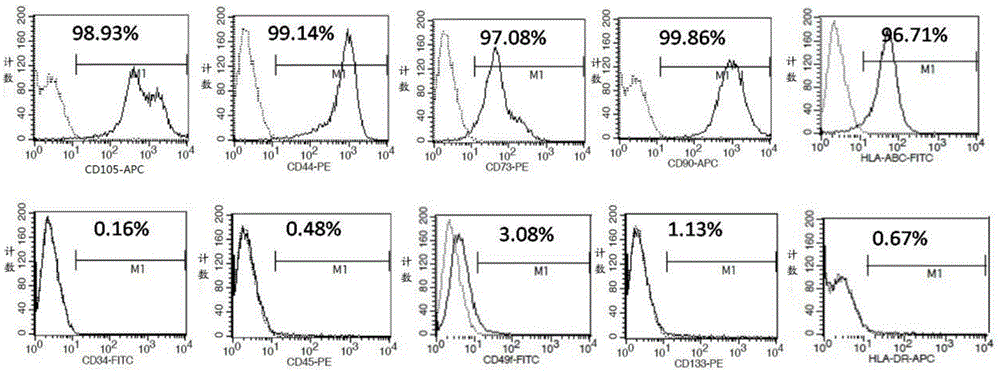
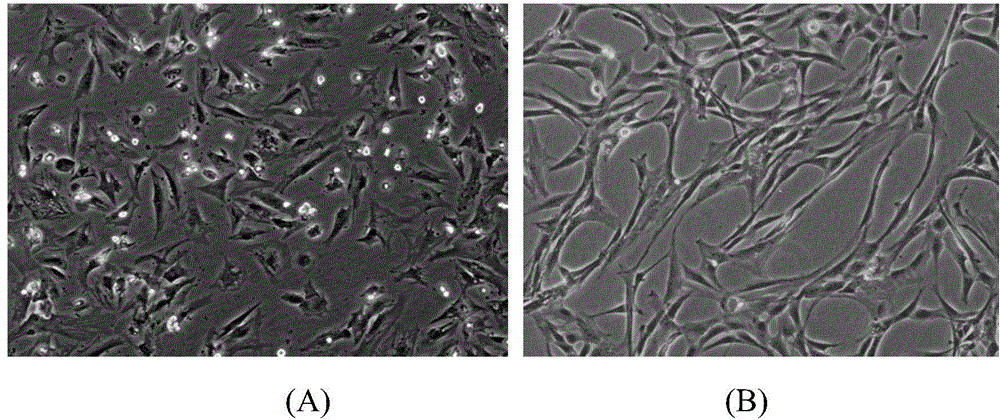
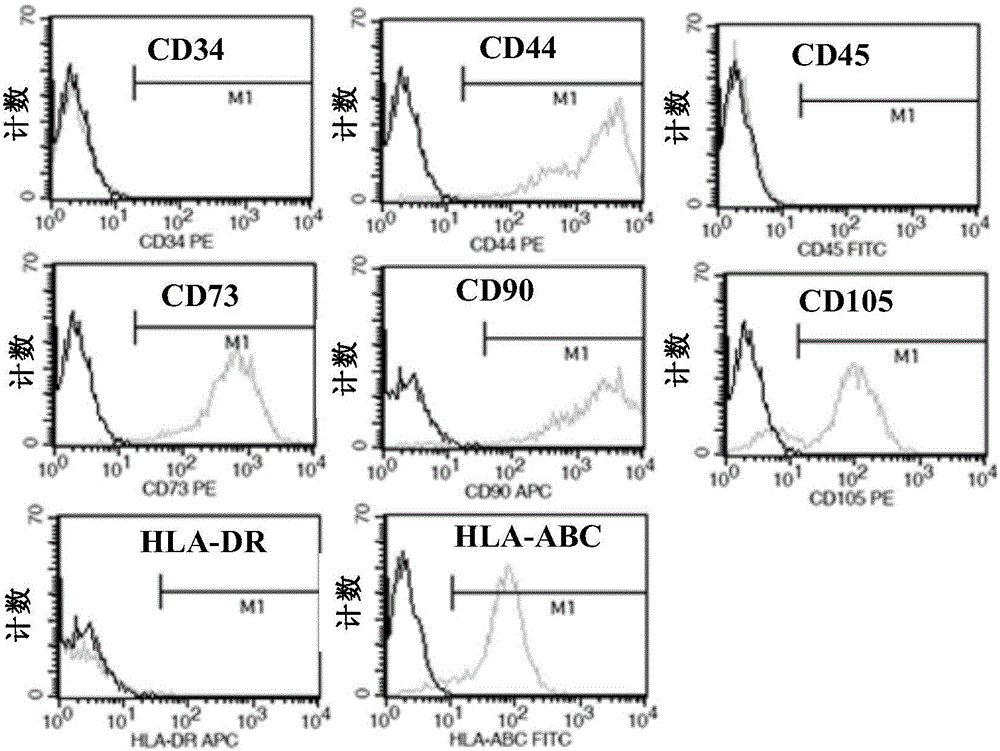
[0053] Example 1: Preparation of umbilical cord mesenchymal stem cells
[0054] The preparation of umbilical cord mesenchymal stem cells comprises the following steps:
[0055] (1) Take fresh umbilical cords taken by cesarean section that have left the mother’s body for no more than 2 hours, and check whether the puerpera is carrying infectious disease pathogens (negative hepatitis B surface antigen, hepatitis C antibody, syphilis antibody, and AIDS antibody), and place the fresh umbilical cord containing D- In the special storage bottle of Hanks balance solution, it is transported in a low-temperature ice box;
[0056] (2) Put the umbilical cord in a biosafety cabinet for ultraviolet irradiation for 30 minutes, preheat the water-bath shaker at 37°C in advance, and place the umbilical cord sample bottle in the biosafety cabinet for operation. Take out the sample with long hemostatic forceps and place it in a 150mm sterile glass dish, and wash away the blood on the surface of ...
Embodiment 2
[0065] Example 2: Preparation of Umbilical Cord Mesenchymal Stem Cell Injection
[0066] Described cryopreservation solution comprises following components:
[0067]
[0068] (1) Take Bomaili A injection, 20% human serum albumin, hydroxyethyl starch 130 / 0.4 and adenosine triphosphate-disodium magnesium chloride freeze-dried powder according to the above formula, and mix them;
[0069] (2) Filter the mixed solution in step (1) with a 0.22 μm bacterial filter, then add the prepared umbilical cord mesenchymal stem cells, and the concentration is controlled at 5×10 6 cells / ml, cell suspension was obtained;
[0070] (3) Add clinical-grade DMSO to the cell suspension in step (2), and pack in In the cell cryopreservation bag, the mesenchymal stem cell injection was obtained.
Embodiment 3
[0071] Example 3: Preparation of Umbilical Cord Mesenchymal Stem Cell Injection
[0072]
[0073] The preparation method was as in Example 2, and the concentration of umbilical cord mesenchymal stem cells was controlled at 8×10 6 pieces / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com