Anticancer hydroxyethyl starch-doxorubicin conjugate and preparation method thereof
A technology of hydroxyethyl starch and doxorubicin, which is applied in the field of biomedicine, can solve the problems of complex preparation process, dangerous operation, toxic and side effects of doxorubicin conjugates, etc., to prolong cycle time, facilitate operation and control, Improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
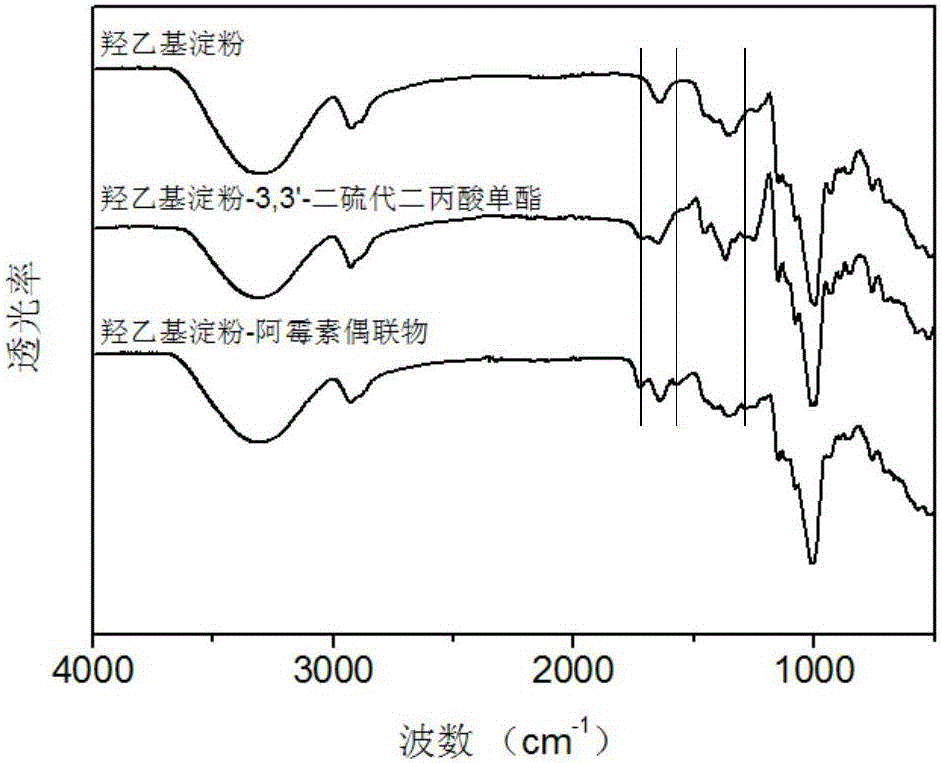
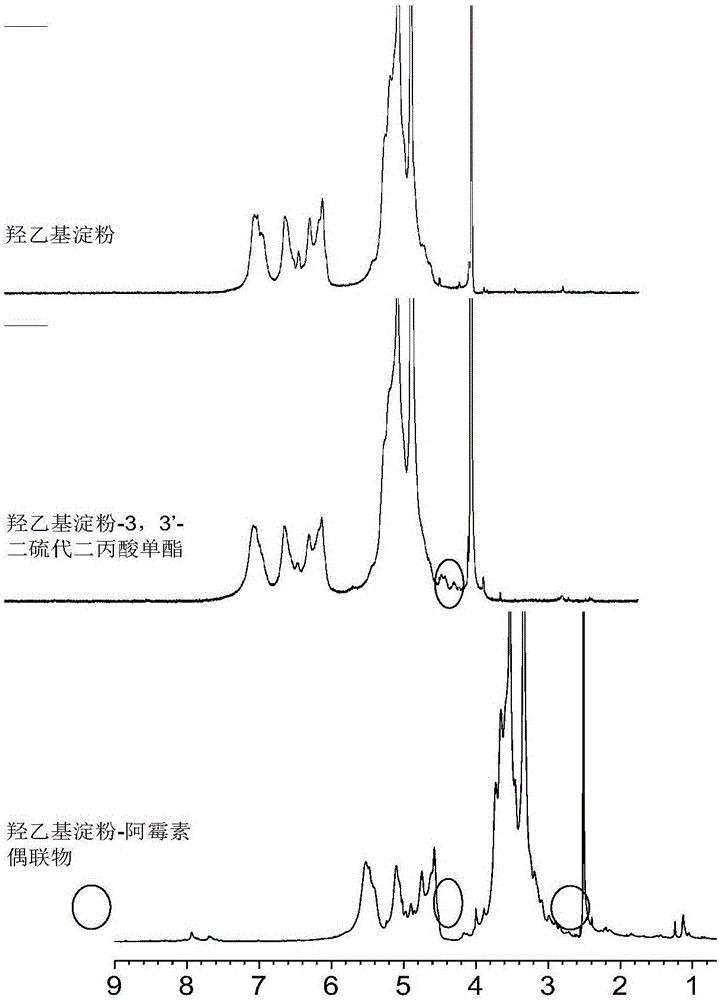
Image
Examples
preparation example Construction
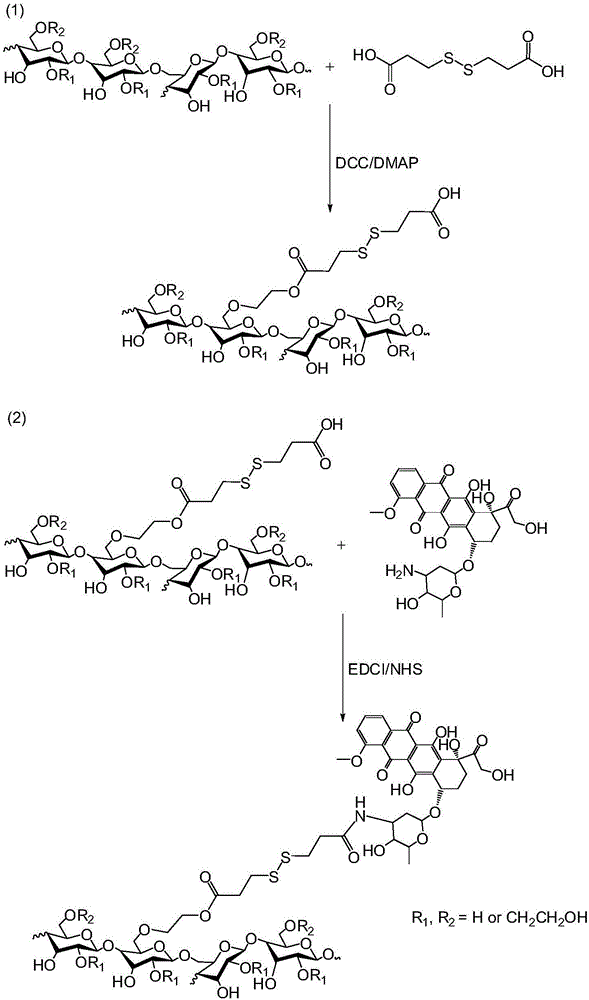
[0060] The preparation method of hydroxyethyl starch-doxorubicin conjugate of the present invention comprises the following steps:
[0061] (1) react hydroxyethyl starch with 3,3'-dithiodipropionic acid in the presence of dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) to generate formula (II ) of hydroxyethyl starch-3,3'-dithiodipropionic acid monoester, wherein R 1 and R 2 for H or CH 2 CH 2 OH;
[0062]
[0063] (2) The hydroxyethyl starch-3,3'-dithiodipropionic acid monoester obtained in step (1) was mixed with doxorubicin in 1-(3-dimethylaminopropyl) under the condition of avoiding light. Under the condition that -3-ethylcarbodiimide hydrochloride (EDCI), N-hydroxysuccinimide (NHS) and triethylamine exist, react the hydroxyethyl starch-doxorubicin shown in formula (I) prime conjugates.
[0064] The preparation method of described hydroxyethyl starch-doxorubicin conjugate specifically comprises the steps:
[0065] (1) Dissolve 3,3'-dithiodipropi...
Embodiment 1
[0079] Prepare the anticancer drug hydroxyethyl starch-doxorubicin conjugate according to the following steps:
[0080] (1) Dissolve 2.96g (14.10mmol) of 3,3'-dithiodipropionic acid in 20mL of dimethylsulfoxide, then add 0.58g (2.82mmol) of dicyclohexylcarbodiimide (DCC), 4- Dimethylaminopyridine (DMAP) 0.17g (1.41mmol), stirred at room temperature for 0.5h to obtain a brown reaction solution A, then added 1.04g of hydroxyethyl starch (sugar ring is 5.65mmol) to the brown reaction solution A, and continued at room temperature Stirring and reacting for 48 hours to obtain reaction solution B, wherein the molecular weight of hydroxyethyl starch is 200kDa, and the degree of substitution of hydroxyethyl is 0.5;
[0081] (2) Filter the reaction solution B to remove the dicyclohexyl urea generated in the reaction, pour the filtrate into 200 mL of ethanol / ether mixed solvent (1:1, V / V), and stir to obtain a suspension C;
[0082] (3) Suspension C was filtered to obtain a brown precip...
Embodiment 2
[0088] (1) Dissolve 2.96g (14.10mmol) of 3,3'-dithiodipropionic acid in 20mL of dimethylsulfoxide, then add 0.58g (2.82mmol) of dicyclohexylcarbodiimide (DCC), 4- Dimethylaminopyridine (DMAP) 0.17g (1.41mmol), stirred at room temperature for 0.5h to obtain a brown reaction solution A, then added 1.04g of hydroxyethyl starch (sugar ring is 5.65mmol) to the brown reaction solution A, and continued at room temperature Stirring and reacting for 48 hours to obtain reaction solution B, wherein the molecular weight of hydroxyethyl starch is 70kDa, and the degree of substitution of hydroxyethyl is 0.5;
[0089] (2) Filter the reaction solution B to remove the dicyclohexyl urea generated in the reaction, pour the filtrate into 200 mL of ethanol / ether mixed solvent (1:1, V / V), and stir to obtain a suspension C;
[0090] (3) Suspension C was filtered to obtain a brown precipitate, which was washed three times with 150 mL of ethanol / ether mixed solvent (1:1, V / V), 50 mL each time, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com