Amphipathy hydroxyethyl-starch-coupled-polylactic-acid copolymer and preparing method and application thereof
A polylactic acid copolymer and hydroxyethyl starch technology, which is applied in the multidisciplinary field, can solve the problems of low drug loading, large size, unstable structure, etc., and achieve the effects of uniform chain length, high degree of polymerization, and stable structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
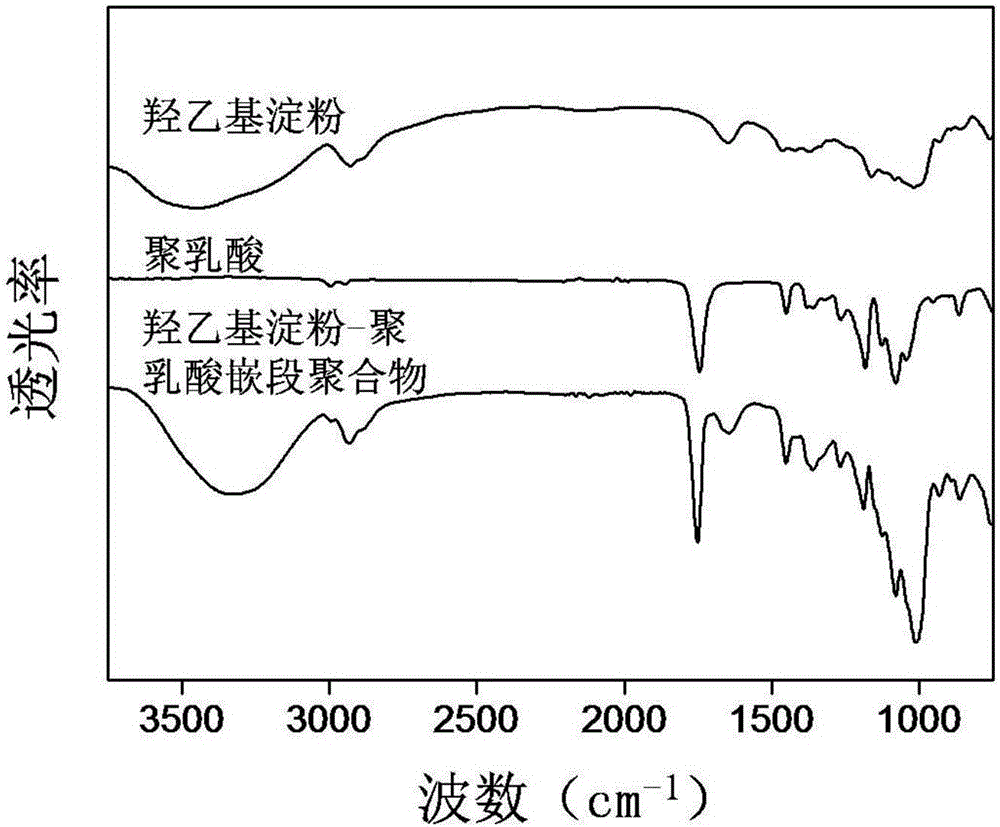
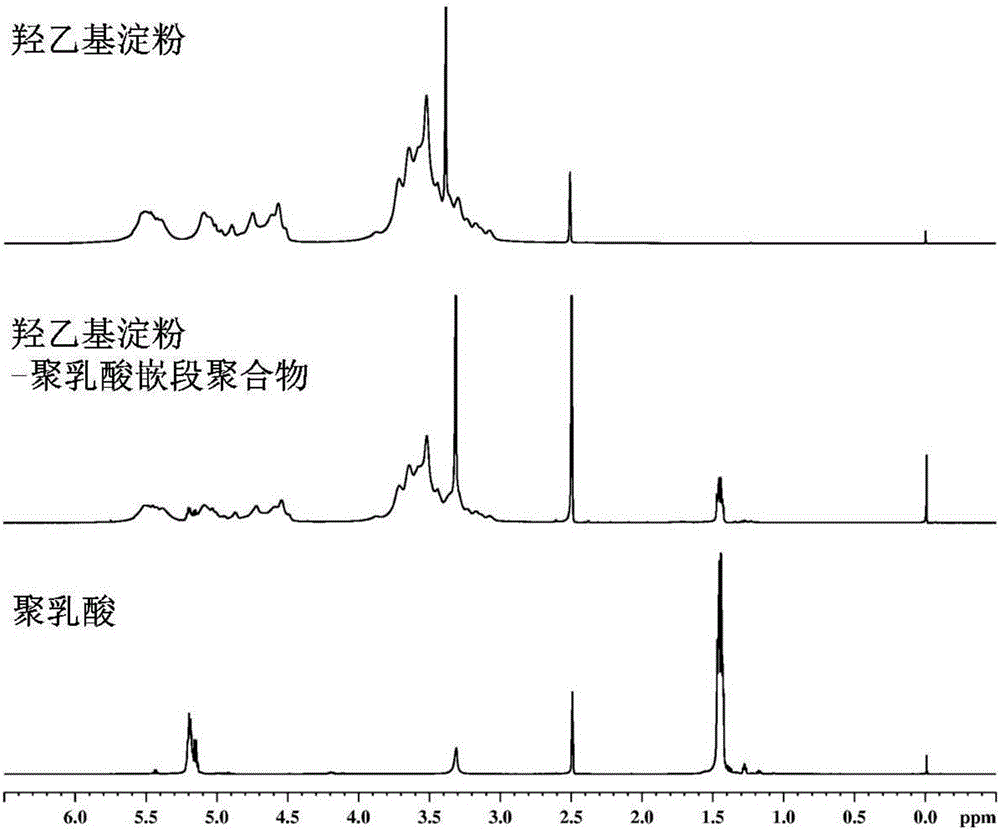
Image
Examples
preparation example Construction
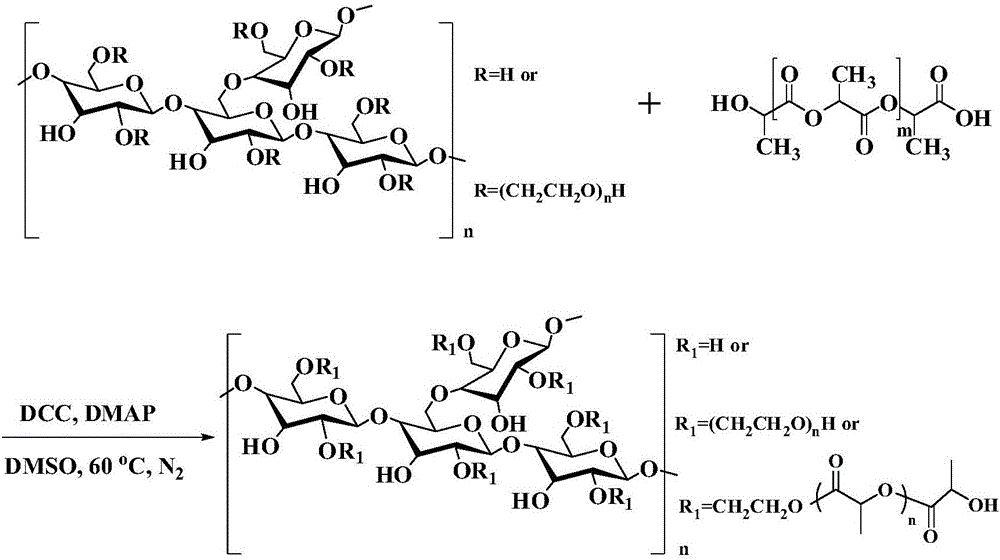
[0040] The preparation method, such as figure 1 As shown, it specifically includes the following steps:
[0041] (1) Dissolving polylactic acid and activating its terminal carboxyl group: Add catalyst N-N'-dicyclohexylcarbodiimide and 4-dimethylaminopyridine to carboxyl-terminated polylactic acid, and use anhydrous dimethyl sulfoxide as a solvent , react at 50-70°C for 25-45 minutes, make it dissolve completely, and obtain polylactic acid activated by the carboxyl-terminated group; said polylactic acid, N-N'-dicyclohexylcarbodiimide and 4-dimethylaminopyridine The feeding molar ratio is 1:4:2;
[0042] (2) Dissolving hydroxyethyl starch: under the condition of nitrogen protection, fully dissolve the hydroxyethyl starch in anhydrous dimethyl sulfoxide at 50-70° C. to obtain a dimethyl sulfoxide solution of hydroxyethyl starch;
[0043] (3) Esterification reaction: the polylactic acid activated by the carboxyl end group obtained in step (1) is mixed with the dimethyl sulfoxide...
Embodiment 1
[0055] A kind of amphiphilic block polymer hydroxyethyl starch-polylactic acid (HES-g-PLA), is synthesized according to the following steps:
[0056] (1) Dissolve the polylactic acid and activate the terminal carboxyl group: 0.565g carboxyl-terminated polylactic acid (PLA-COOH), N-N'-dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP ), wherein the molar ratio of PLA, DCC, and DMAP is 1:4:2 and placed in a 100mL dry round bottom flask, the system is replaced with nitrogen, and a nitrogen balloon is connected. At the same time, 15 mL of anhydrous dimethyl sulfoxide (DMSO) solvent under nitrogen protection was added, and stirred at 60° C. for 30 min to completely dissolve the reactant and activate its terminal carboxyl group.
[0057] (2) Dissolving hydroxyethyl starch: Dissolve 0.5 g of hydroxyethyl starch in 10 mL of anhydrous DMSO solvent while performing step (1). The whole reaction process uses nitrogen as a protective gas, and the reaction temperature is set to ...
Embodiment 2
[0065] A kind of amphiphilic block polymer hydroxyethyl starch-polylactic acid (HES-g-PLA), is synthesized according to the following steps:
[0066] (1) Dissolve polylactic acid and activate the terminal carboxyl group: 0.323g carboxyl-terminated polylactic acid (PLA-COOH), N-N'-dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP ), wherein the molar ratio of PLA, DCC, and DMAP is 1:4:2 and placed in a 100mL dry round bottom flask, the system is replaced with nitrogen, and a nitrogen balloon is connected. At the same time, 15 mL of anhydrous dimethyl sulfoxide (DMSO) solvent under nitrogen protection was added, and stirred at 50° C. for 25 min to completely dissolve the reactant and activate its terminal carboxyl group.
[0067] (2) Dissolving hydroxyethyl starch: Dissolve 0.5 g of hydroxyethyl starch in 10 mL of anhydrous DMSO solvent while performing step (1). The whole reaction process uses nitrogen as a protective gas, and the reaction temperature is set at 50 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com