Liquid-state lipid micro-particles used for delivering cerebric medicine through olfactory pathway, preparation method thereof, and preparation thereof
A technology of liquid lipids and microparticles, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problem of unfavorable processing conditions for hormones, peptides, proteins, genes or vaccines, drug stability, long freeze-drying process, and inability to guarantee high quality. Encapsulation efficiency and other issues to achieve the effect of promoting drug absorption, avoiding first-pass effect, and prolonging drug action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
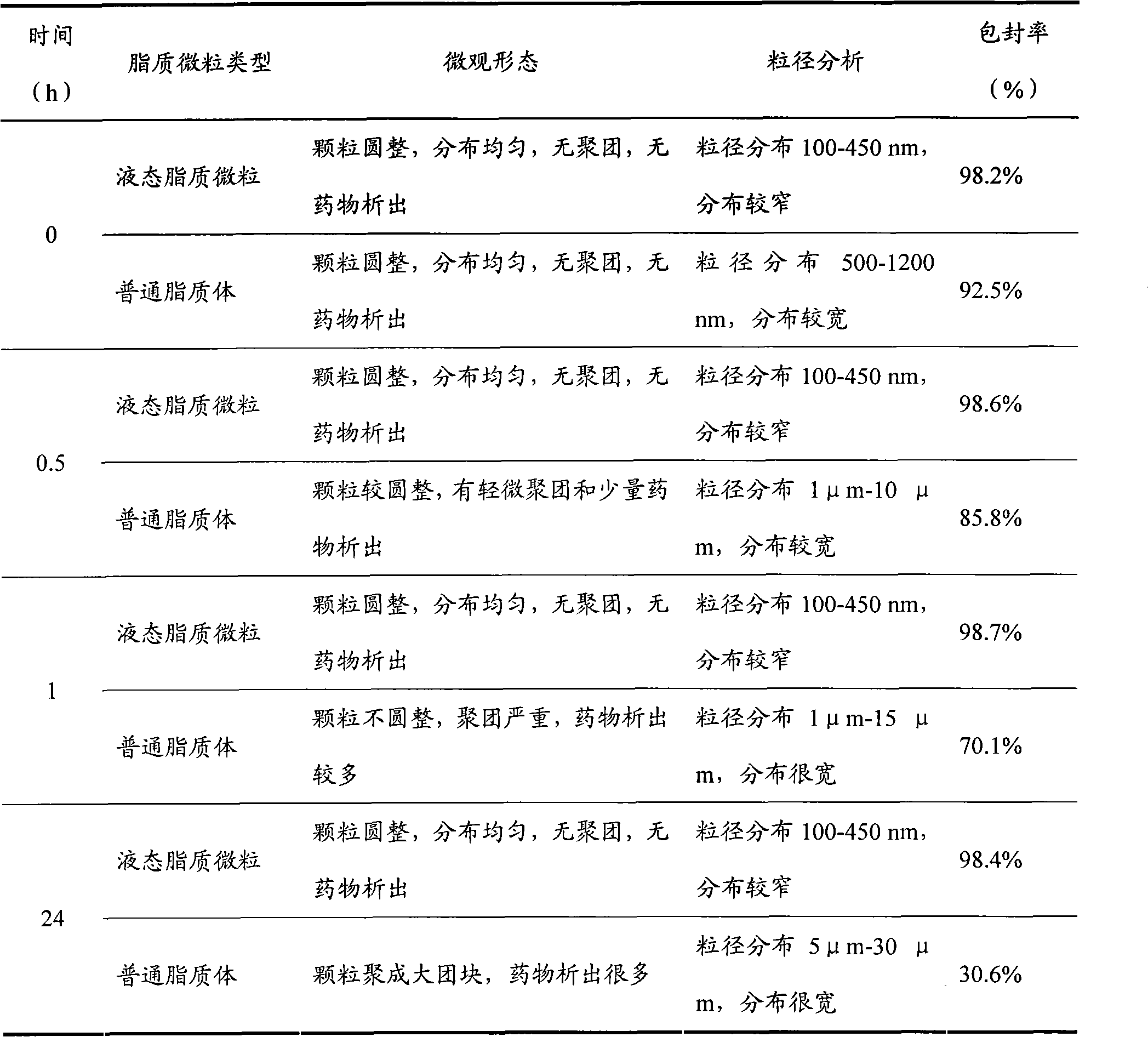
[0024] Many traditional Chinese medicine plants such as Melaleuca pagoda, Yizhiren, Polygala, ginseng, celery seed, safflower, etc. contain active ingredients for treating stroke and senile dementia. However, due to the lack of brain selectivity of common Chinese medicine preparations such as decoctions, tablets, capsules, and injections, the toxicity and side effects are large during application, and the therapeutic effect is poor. In the first embodiment of the present invention, Huperzine A, the active ingredient of Melaleuca plant alkaloids, is used as the main drug, ethanol and Tween 80 are used as penetration enhancers, and the natural phospholipid material soybean phospholipid and cholesterol are used as the main lipid materials to prepare liquid Lipid particles.
[0025] Labeling of huperzine A: reference [Shi Senlin, Xu Lianying, Wu Jinjin, etc. Comparison of drug distribution of breviscapine in different routes of administration in the brain. Acta Pharmaceutica Sinic...
Embodiment 2
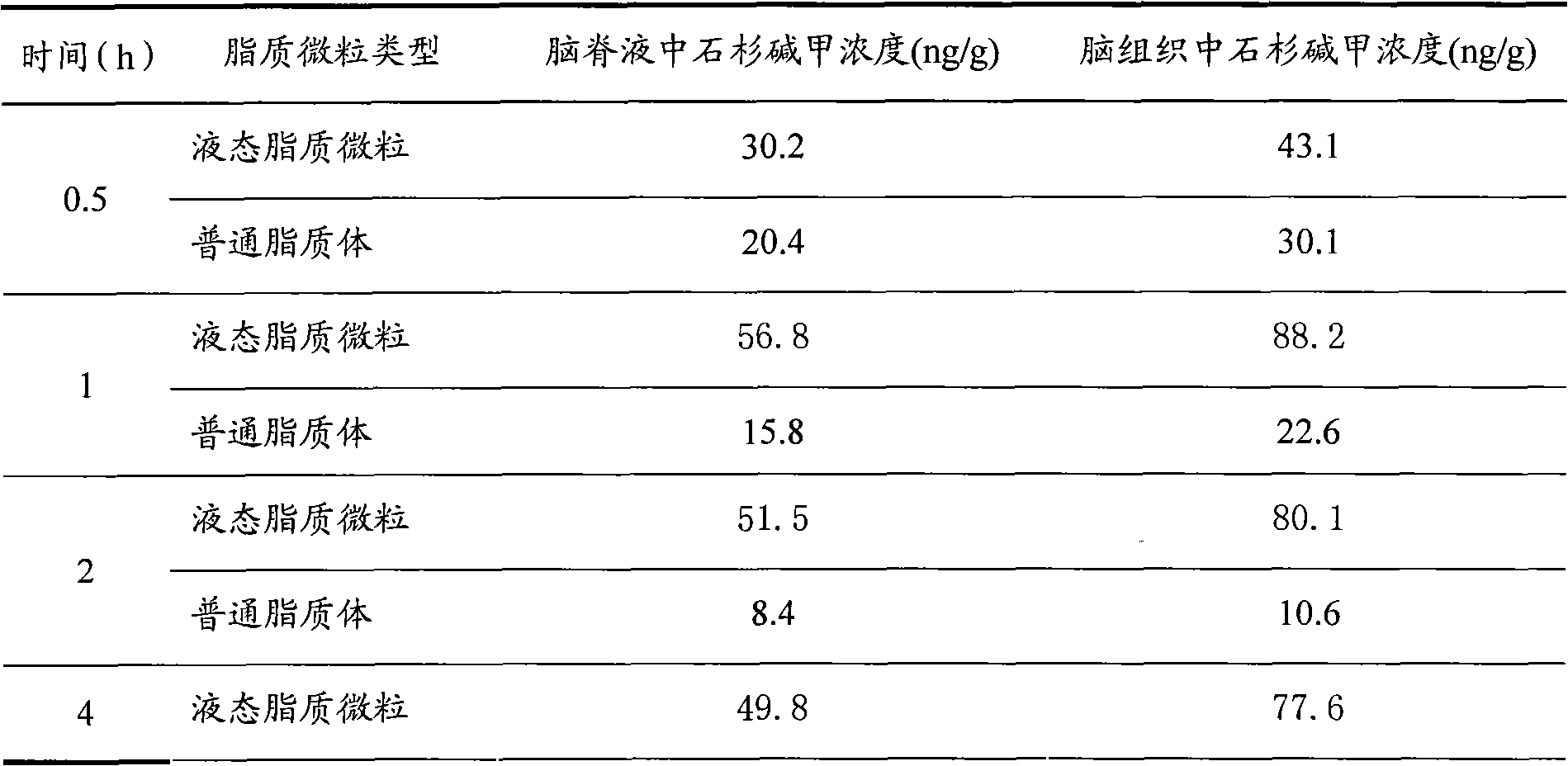
[0034] The second embodiment of the present invention utilizes two kinds of lipid particles loaded with huperzine A prepared in Example 1 to carry out animal experiments, and compares the ordinary liposomes and liquid lipid particles loaded with huperzine A to promote drug entry. brain delivery efficiency.
[0035] Experimental method: SD rats were anesthetized by intraperitoneal injection of 20% urethane (5ml / kg), fixed in supine position, the head was slightly elevated, the neck was opened, the trachea and esophagus were exposed, the trachea was intubated to maintain the normal breathing of the rat, and acrylic acid was applied. Resin seals the pharynx. Insert rat nostril about 5mm with micro-injector, slowly push into each 0.5ml of the lipid particle solution of two kinds of encapsulating huperzine A prepared in embodiment 1, after administration, at 0.5, 1, 2 and 4h (each time Point 3 rats) to collect cerebrospinal fluid and brain tissue samples to determine the drug cont...
Embodiment 3
[0043] Biomacromolecular drugs such as hormones, peptides, genes or vaccines have the characteristics of large molecular weight, poor thermal stability, and easy to be rapidly degraded in the body. The nasal olfactory pathway enters the brain. In the third embodiment of the present invention, synthetic phospholipids are used as materials, insulin is used as a model drug, combined with solid dispersion technology to improve the dissolving ability of insulin in propylene glycol, and filtered through a 0.2 μm filter membrane to prepare insulin liquid lipid particles below 250 nm.
[0044] Labeling of Insulin: Reference [Zhou Wenli, Yan Chaoying, Zhang Jiantao, Zhao Jing. Exogenous nerve growth factor on blood-brain barrier permeability and brain tissue distribution of neonatal rats with hypoxic brain injury. Chinese Journal of Biological Products. 2010, 23(3): 261-263], using chloramine T method to label insulin, made 125 I - Insulin.
[0045] package 125 The preparation of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com