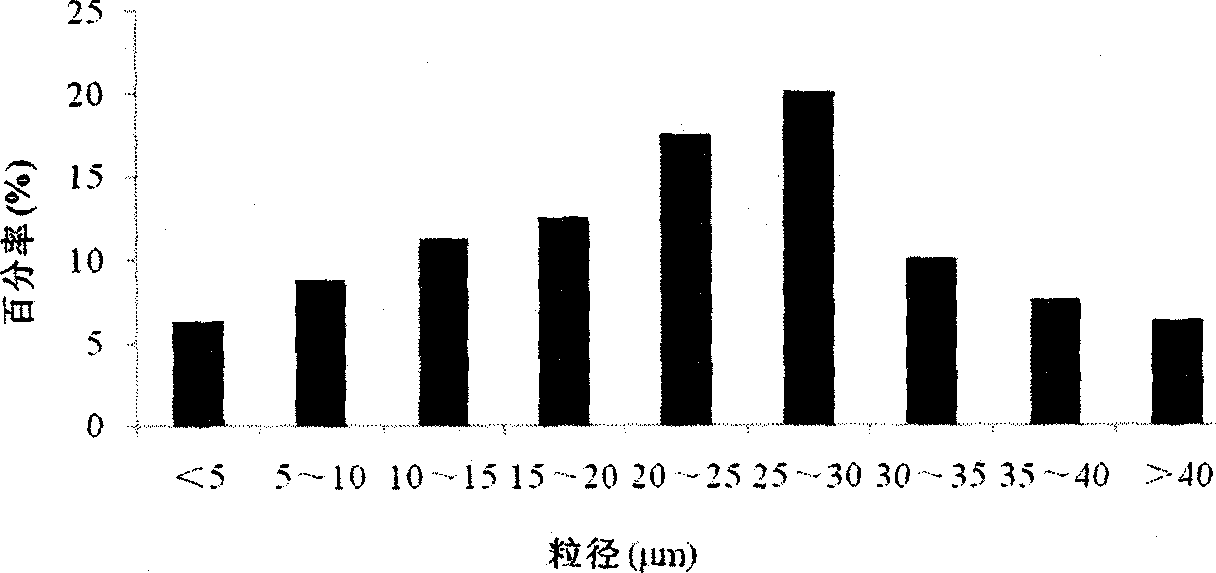
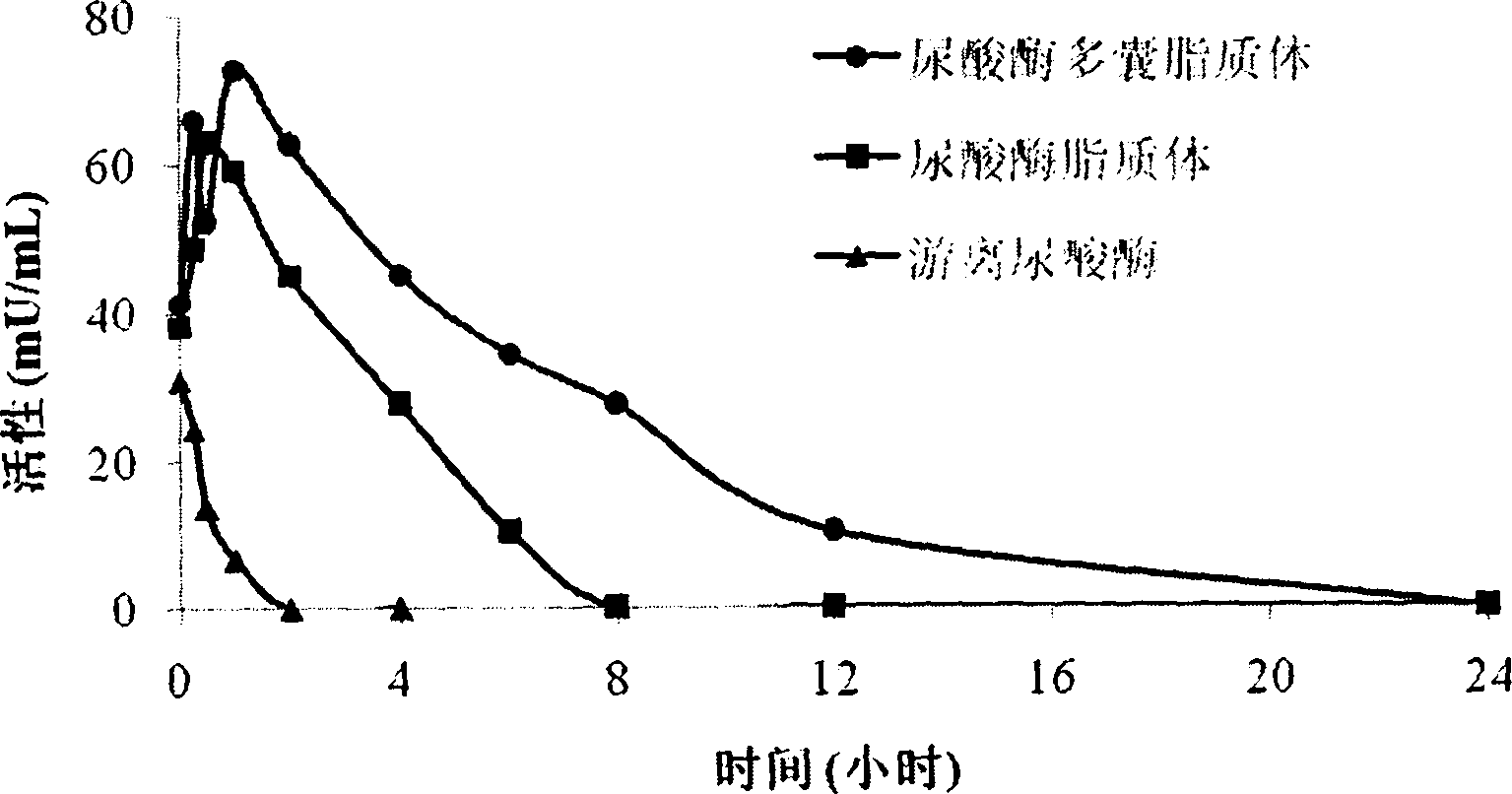
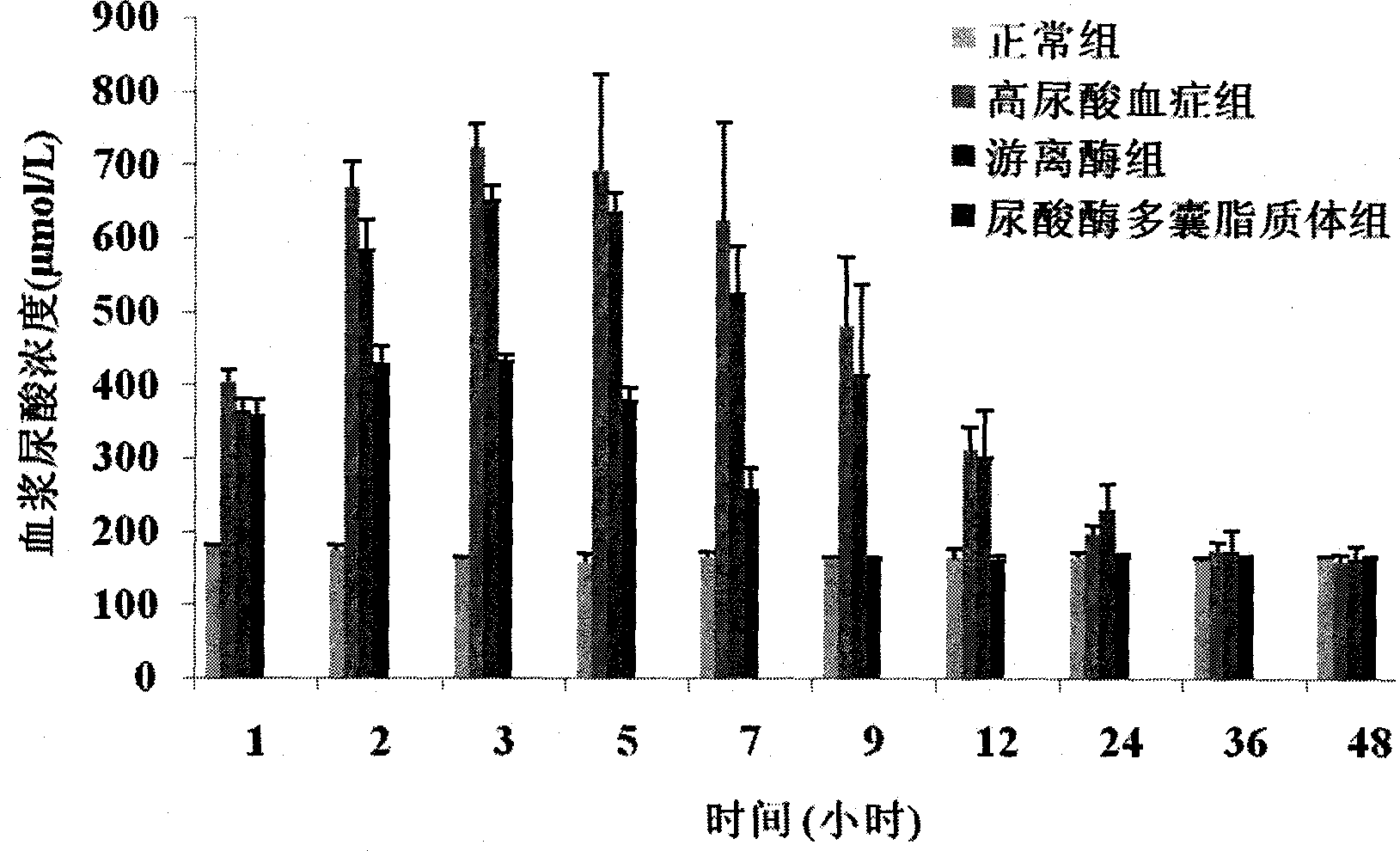
Uricase multivesicular liposome and preparation method thereof
A multivesicular liposome and uricase technology, which can be used in liposome delivery, pharmaceutical formulations, peptide/protein components, etc., can solve the problem of low uricase activity, easy induction of immune responses, and immunogenicity and antigenic reactions And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The composition ratio by weight of each component contained in the formula is: 1 part of lecithin, 1 part of cholesterol, 1 part of triolein, 2 parts of glucose (I), 3.4 parts of poloxamer F-68, poloxamer F-12715 parts, borate buffer pH8 (I) 80 parts, L-lysine 1 part, glucose (II) 10 parts, borate buffer pH8 (II) 200 parts.
[0018] The preparation method comprises the following steps: (1) preparation of colostrum: get uricase, poloxamer F-127, poloxamer F-68, glucose (I) of prescription quantity and be dissolved in the borate buffer solution of prescription quantity In (I), the phospholipids, cholesterol, and triolein of the prescribed amount are dissolved in ether as the oil phase, and the borate buffer (I) is mixed with the oil phase, and W / O is prepared under vortex conditions. Type colostrum; (2) double milk preparation: get the borate buffer solution (II) that the L-lysine of prescription quantity and glucose are dissolved in prescription quantity, add colostrum t...
Embodiment 2
[0020] The composition ratio by weight of each component contained in the formula is: 1.3 parts of lecithin, 1.4 parts of cholesterol, 1.3 parts of triolein, 4 parts of glucose (I), 4.8 parts of poloxamer F-68, poloxamer F-12721 parts, borate buffer pH8.2 (I) 95 parts, L-lysine 2.2 parts, glucose (II) 12 parts, Bicine buffer pH8.7 (II) 360 parts.
[0021] The preparation method is basically the same as in Example 1.
Embodiment 3
[0023] The composition ratio by weight of each component contained in the formula is: 1.6 parts of lecithin, 1.6 parts of cholesterol, 1.5 parts of triolein, 5.5 parts of glucose (I), 6.5 parts of poloxamer F-68, poloxamer F-12728 parts, borate buffer pH8.9 (I) 115 parts, L-lysine 1.2 parts, glucose (II) 10.5 parts, Tris buffer pH8.3 (II) 230 parts.
[0024] The preparation method is basically the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com