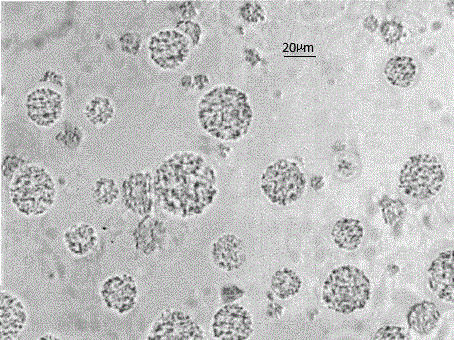
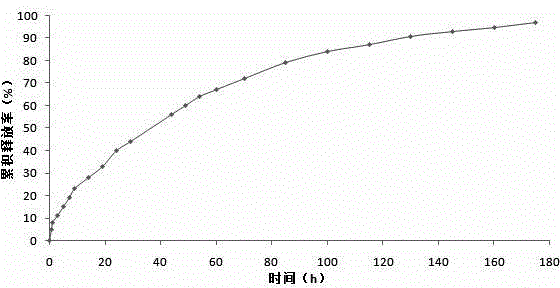
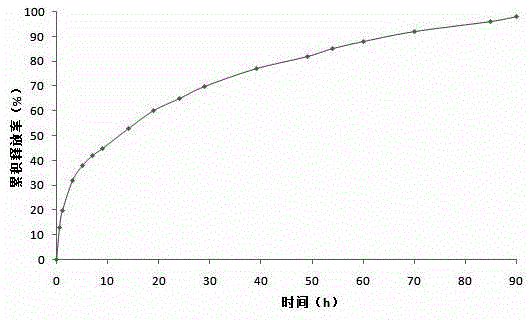
Breviscapine internal phase gelating polycystic lipidosome freeze-dried powder and preparation method thereof
A multivesicular liposome and breviscapine technology, which is applied in liposome delivery, freeze-drying delivery, powder delivery, etc., can solve the problems of short injection half-life, poor stability, poor absorption, etc., to delay drug release and reduce Fluidity, effect of reducing vesicle rupture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of breviscapine internal phase gelled polyvesicular liposome freeze-dried powder
[0039] Weigh 40 mg of phosphatidylcholine, 8.2 mg of triolein, 40 mg of cholesterol, add chloroform-ether (1:1) to dissolve in 2 ml, and use it as the lipid phase; weigh 400 mg of Poloxamer 407, 400 mg of Poloxamer 188 40mg, breviscapine 40mg, sucrose 40mg, arginine 8.7mg, add water to 2ml, adjust pH7.0 to dissolve, as the inner water phase; weigh 5.8mg lysine, glucose 30mg, add water to 4ml, as the outer water phase . Slowly add the inner water phase to the upper layer of the lipid phase, and shear for 8 minutes under the condition of a high-speed shear homogenizer at 10,000 rpm to form a water-in-oil emulsion; Cut down for 8 minutes to form a water-in-oil-in-water type double emulsion; blow dry the double emulsion with nitrogen to remove chloroform and ether therein, and obtain the scutellarin multivesicular liposome suspension; the obtained scutellarin multivesic...
Embodiment 2
[0041] Example 2 Preparation of breviscapine internal phase gelled multivesicular liposome lyophilized powder
[0042] Weigh soybean lecithin 38mg, triolein 10mg, dipalmitoylphosphatidylglycerol 10mg, cholesterol 40mg add chloroform-ether (1:1) to dissolve in 2ml, as lipid phase; weigh poloxamer 407 440mg, Poloxamer 188 70mg, breviscapine 40mg, sucrose 5mg, arginine 10.5mg, add water to 2ml, adjust pH7.0 to dissolve, as the internal water phase; weigh lysine 6mg, glucose 50mg, add water to 4ml, as the external aqueous phase. Slowly add the inner water phase to the upper layer of the lipid phase, and shear for 8 minutes under the condition of a high-speed shear homogenizer at 10,000 rpm to form a water-in-oil emulsion; Cut down for 8 minutes to form a water-in-oil-in-water type double emulsion; blow dry the double emulsion with nitrogen to remove chloroform and ether therein, and obtain the scutellarin multivesicular liposome suspension; the obtained scutellarin multivesicular...
Embodiment 3
[0044] Example 3 Preparation of breviscapine internal phase gelled multivesicular liposome lyophilized powder
[0045] Weigh soybean lecithin 47mg, tricaprylic glyceride 6mg, cholesterol 10mg add chloroform-ether (1:1) to dissolve in 2ml, as lipid phase; weigh poloxamer 407 380mg, poloxamer 188 10mg, scutellaria Add 40 mg of sucrose, 40 mg of sucrose, and 6.5 mg of histidine to 2 ml of water, adjust the pH to 7.0 to dissolve, and use it as the inner water phase; weigh 4 mg of polyvinyl alcohol, 20 mg of glucose, add water to 4 ml, and use it as the outer water phase. Slowly add the inner water phase to the upper layer of the lipid phase, and shear for 8 minutes under the condition of a high-speed shear homogenizer at 10,000 rpm to form a water-in-oil emulsion; Cut down for 8 minutes to form a water-in-oil-in-water type double emulsion; blow dry the double emulsion with nitrogen to remove chloroform and ether therein, and obtain the scutellarin multivesicular liposome suspensio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com