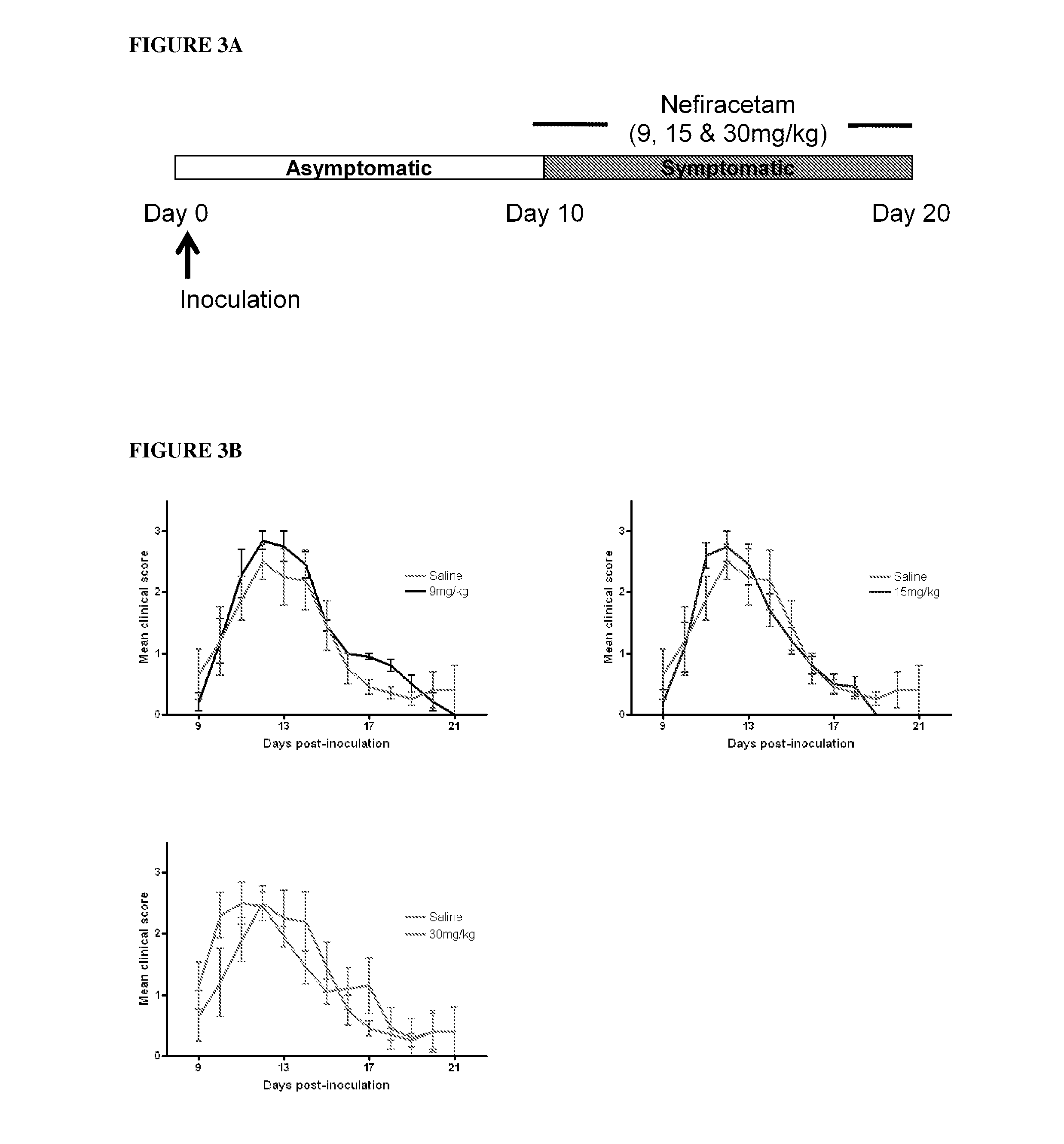
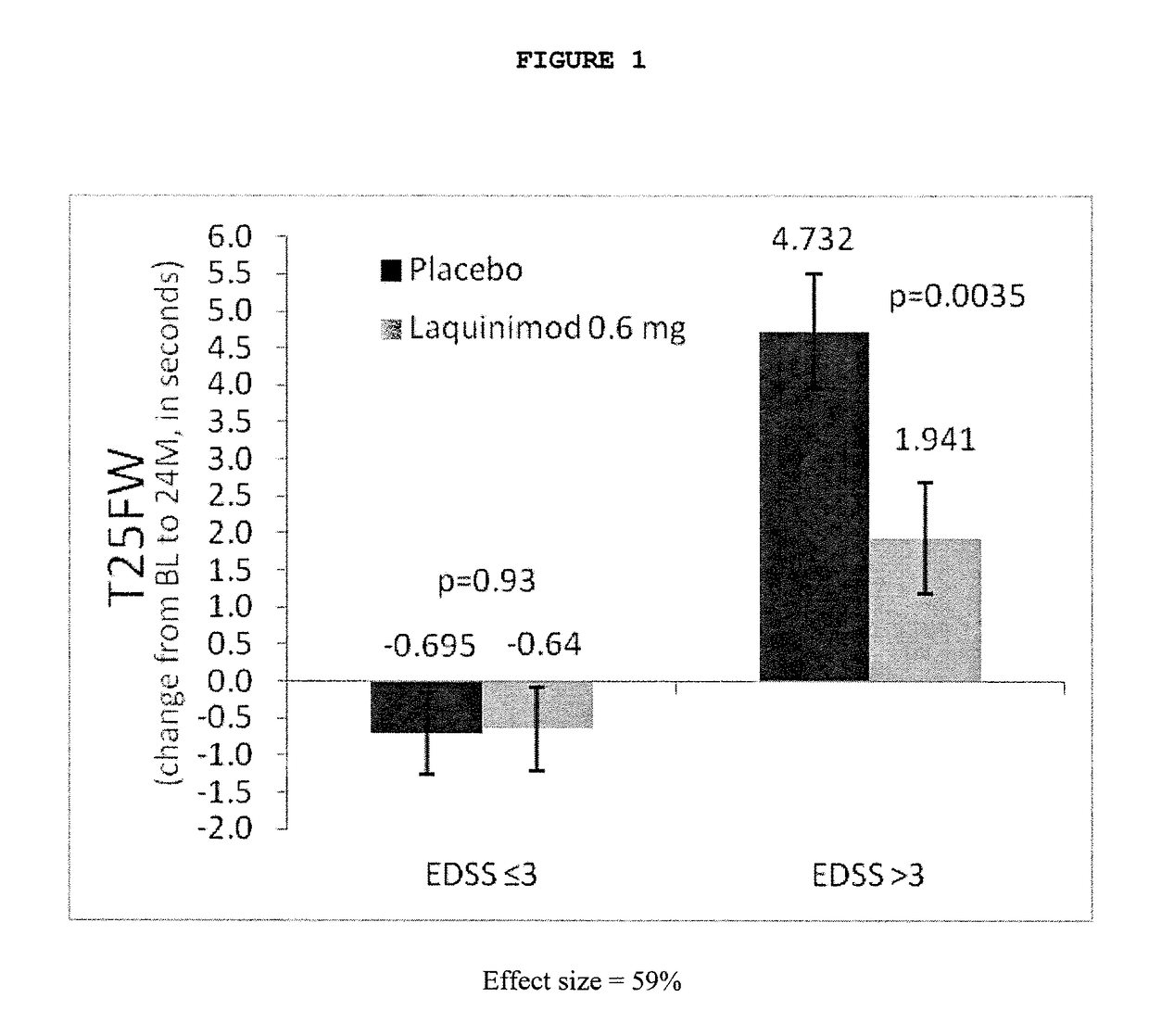
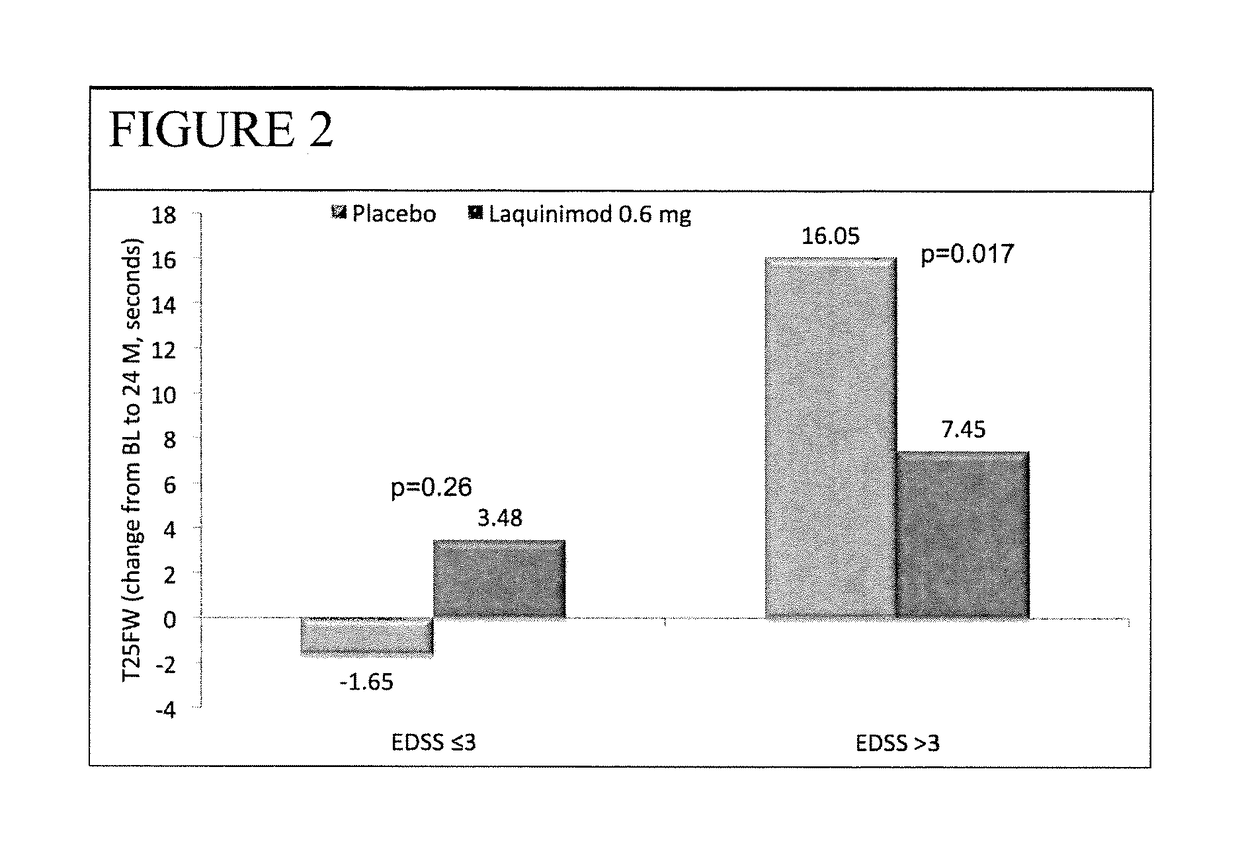
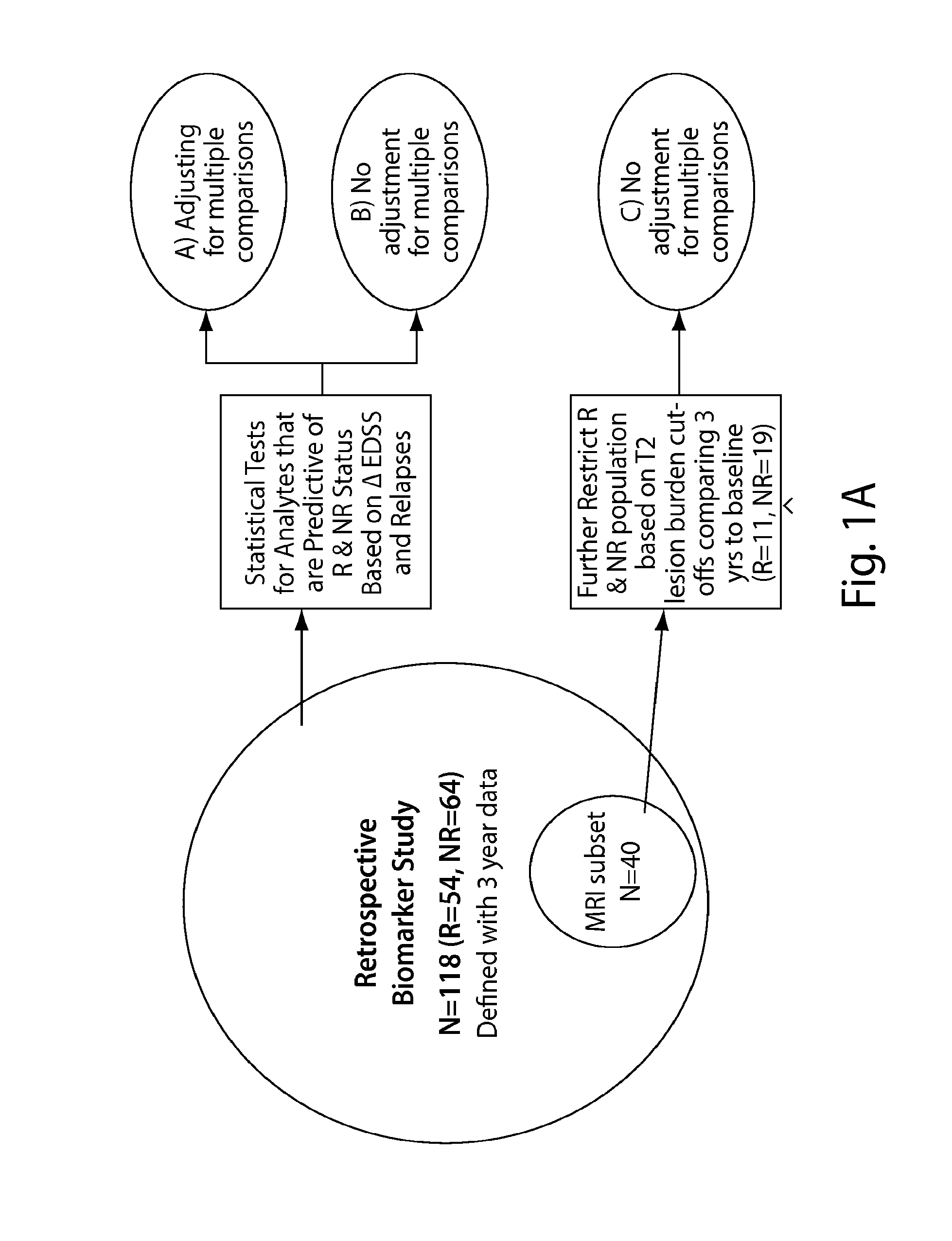
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Relapsing remitting" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Relapsing-remitting MS is defined as MS in which patients have relapses of MS and periods of stability in between relapses. Relapses are episodes of new or worsening symptoms not caused by fever or infection and that last more than 48 hours. In other words, a stable course is punctuated by episodes of new or worse symptoms.
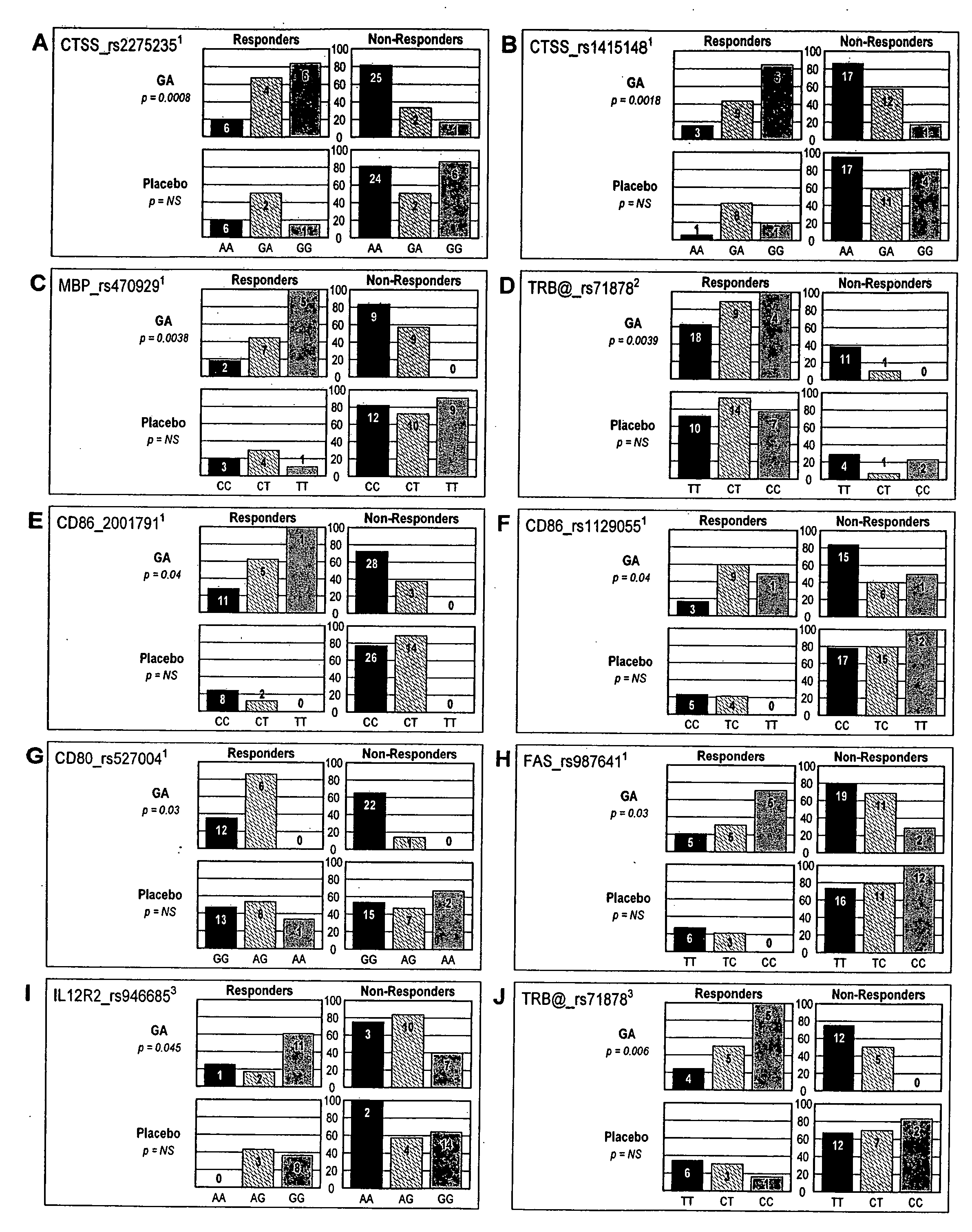
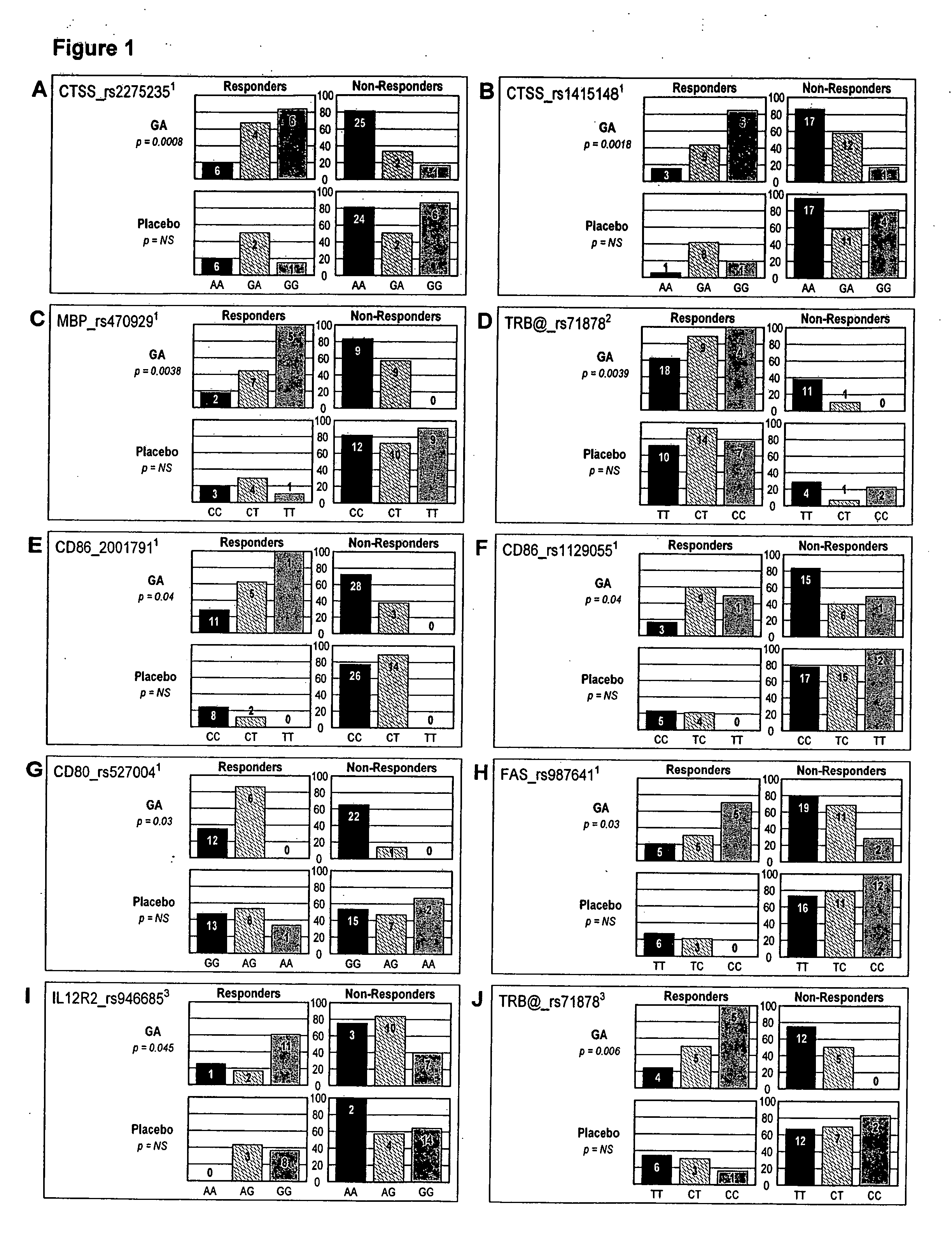
Markers associated with the therapeutic efficacy of glatiramer acetate
The present invention is directed to methods and kits based, at least in part, on the identification of allele-specific responsiveness or non-responsiveness to glatiramer acetate for the treatment of immune disorders, such as relapsing-remitting multiple sclerosis. The allele-specific responsiveness or non-responsiveness is based on polymorphisms in the following genes, CTSS, MBP, TCRB, CD95, CD86, IL-1R1, CD80, SCYA5, MMP9, MOG, SPP1 and IL-12RB2.
Owner:RAPPAPORT FAMILY INSTITUTE FOR RESEACH IN THE MEDICAL SCIENCES +2
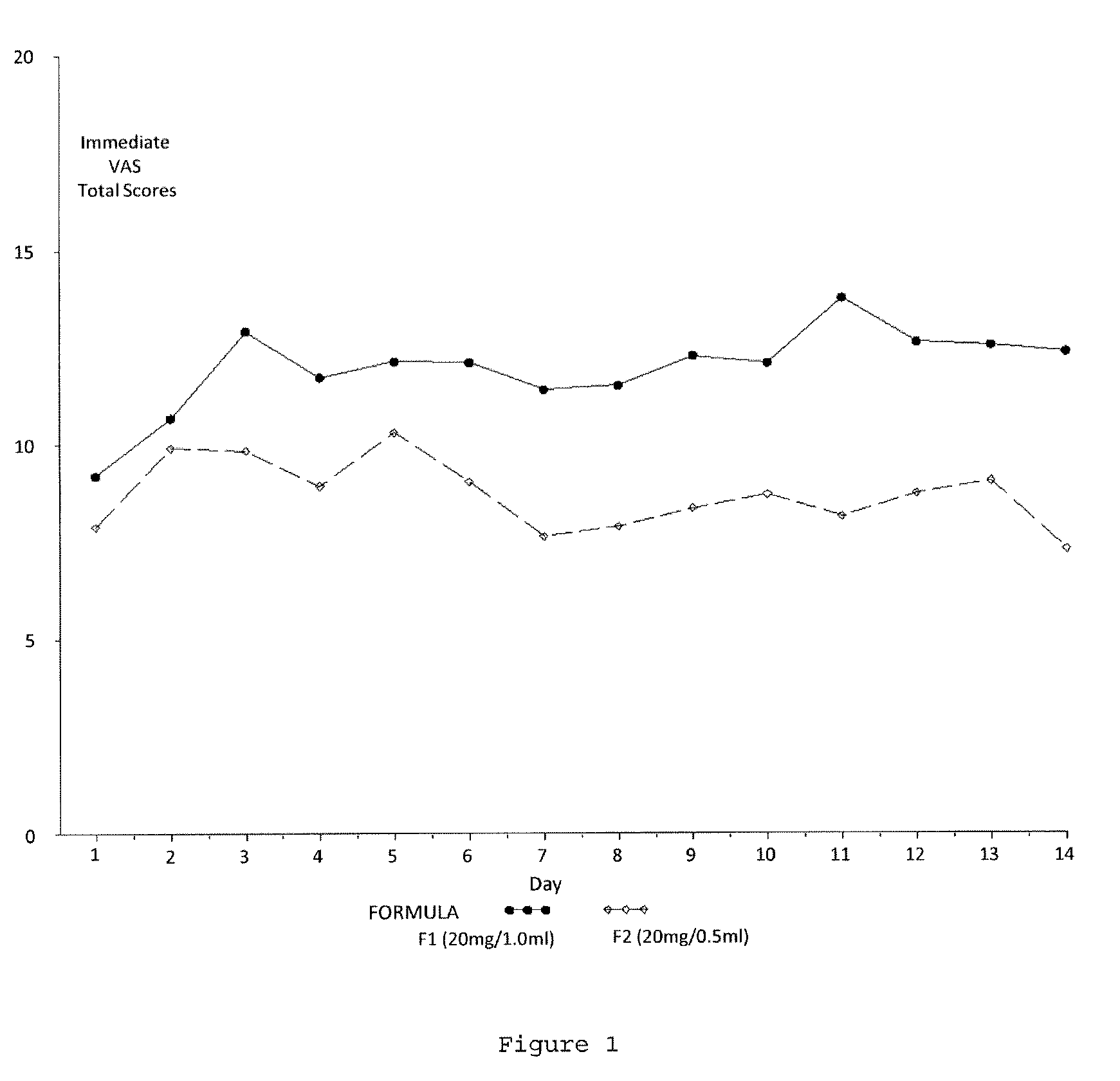
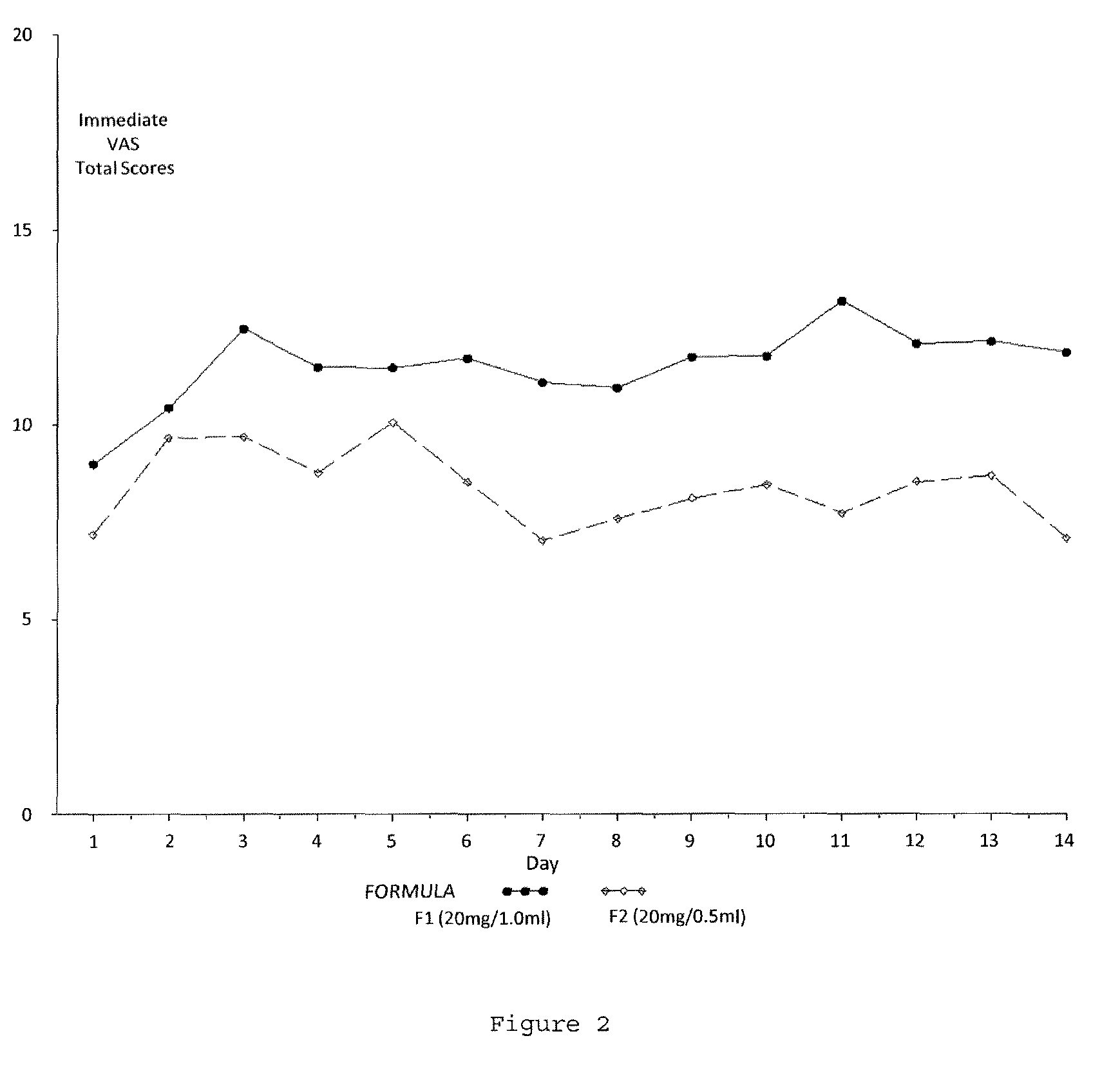
Reduced volume formulation of glatiramer acetate and methods of administration
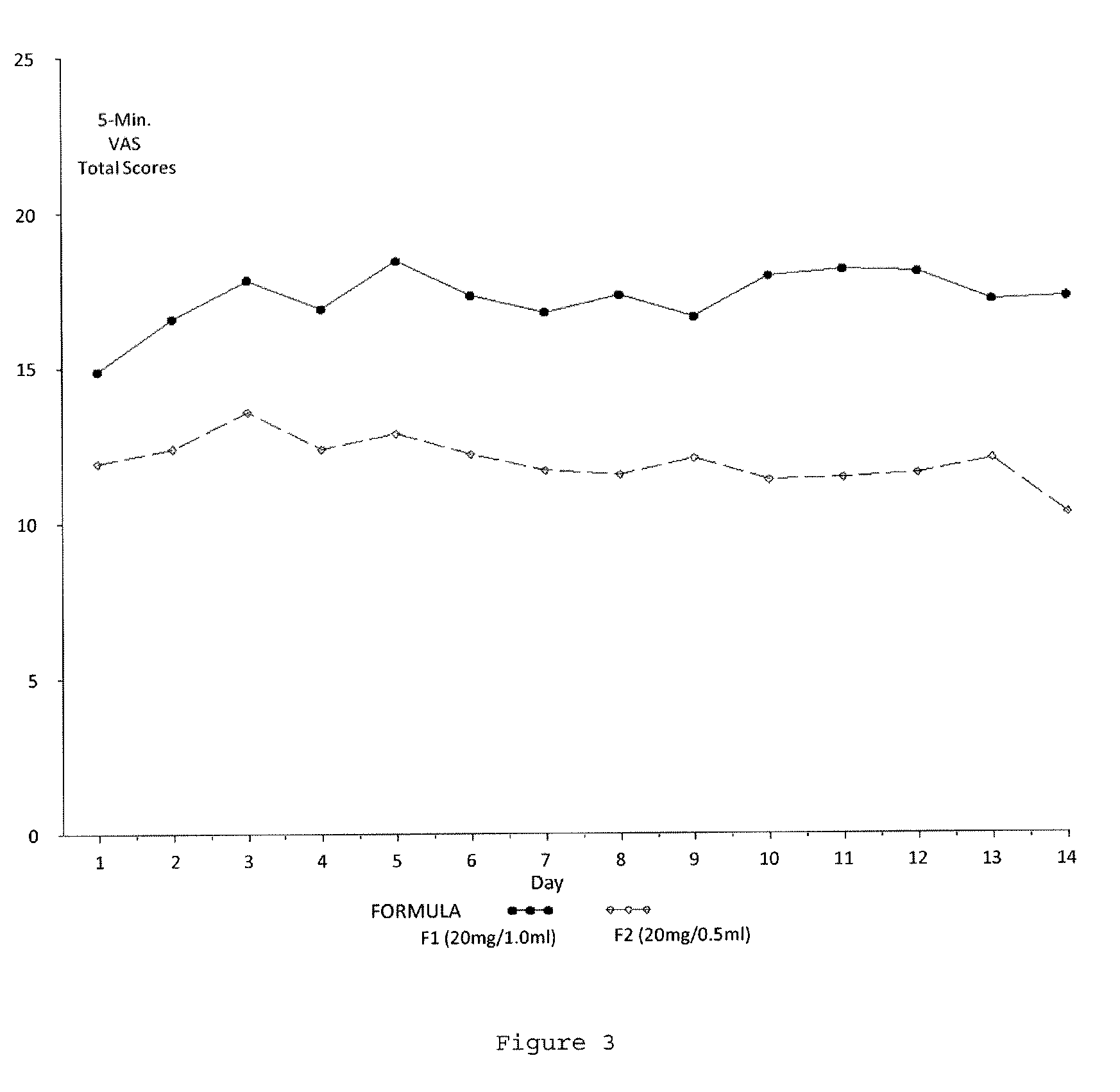
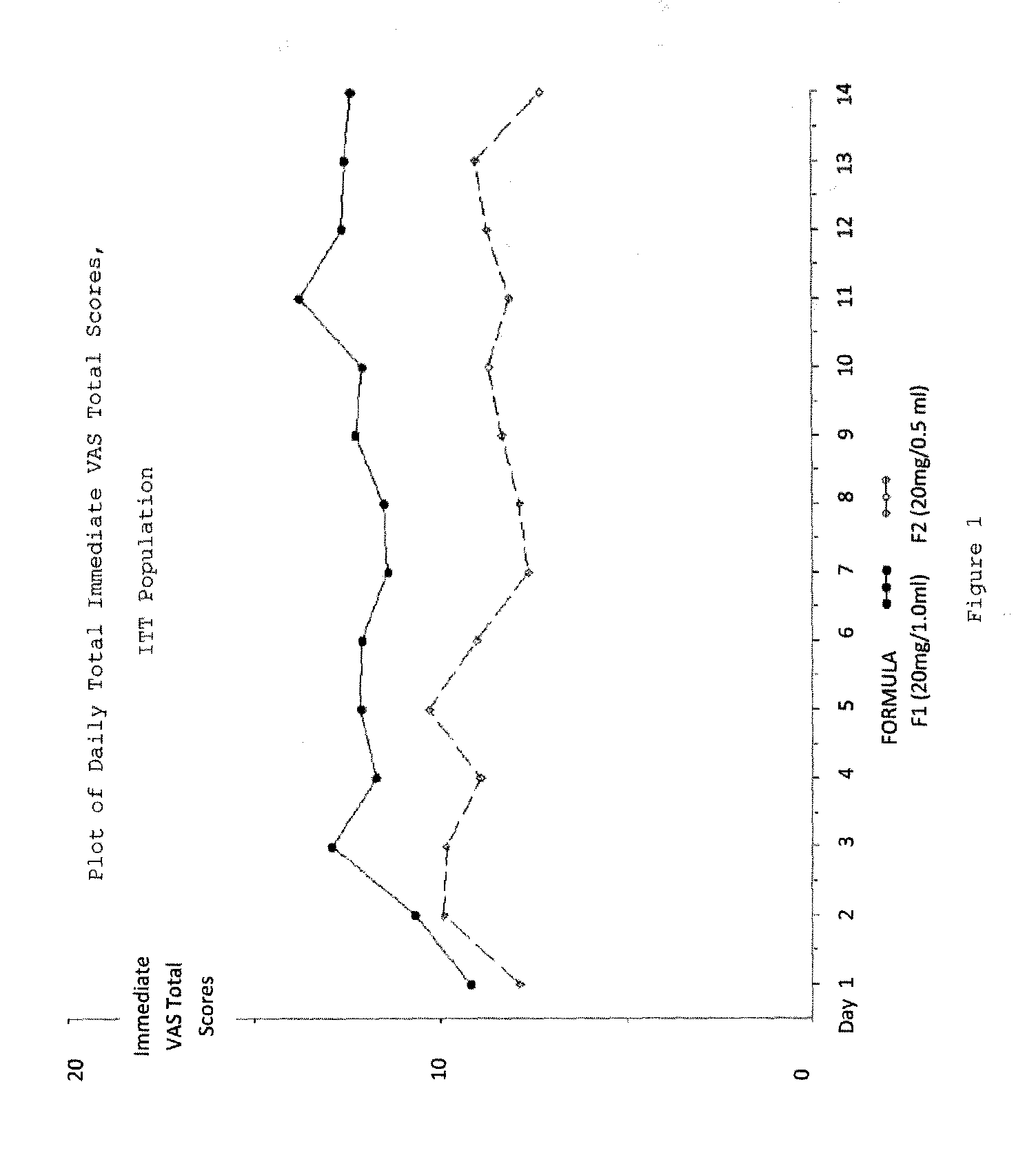
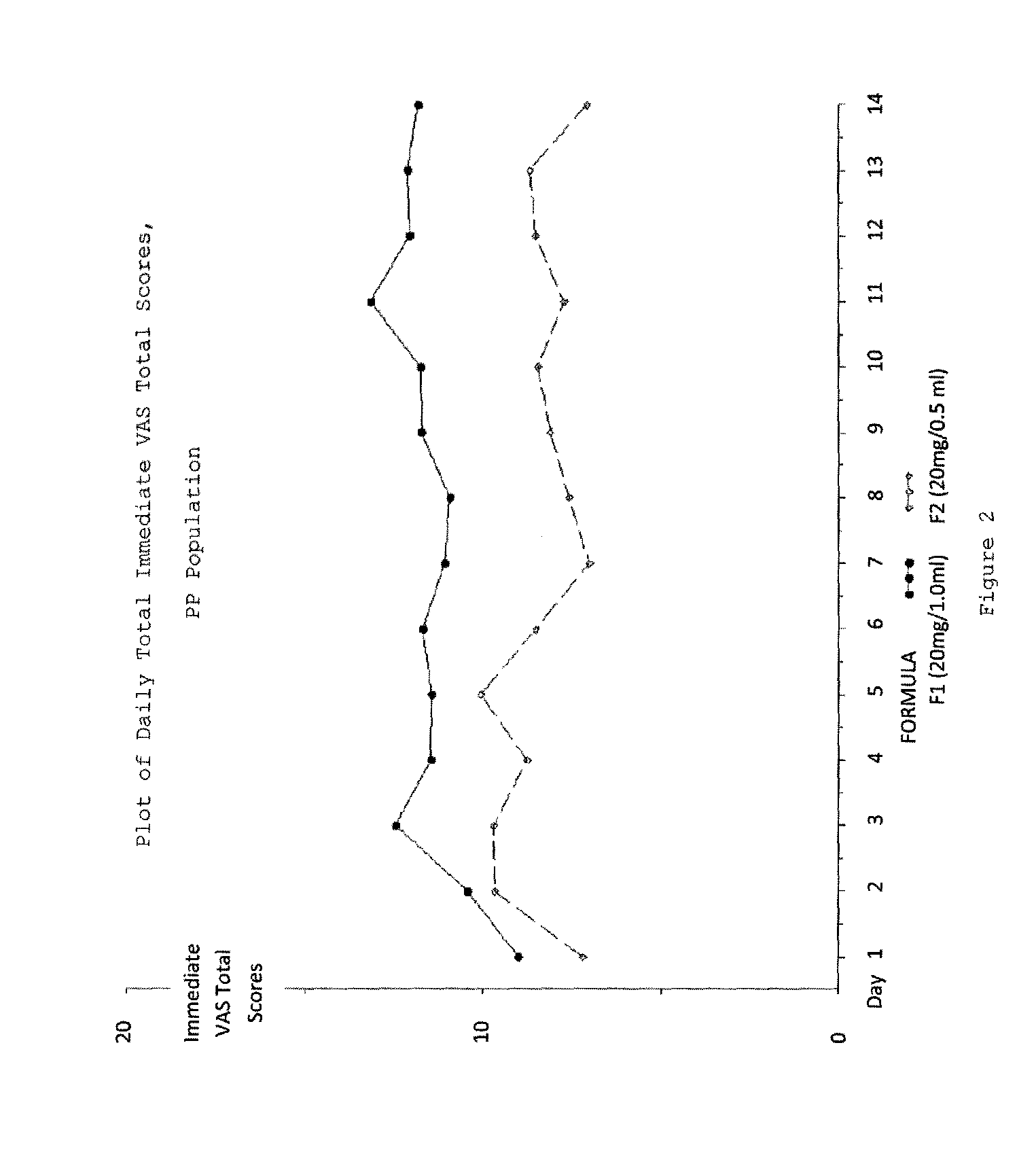
A method for reducing frequency of relapses in a human patient afflicted with relapsing-remitting multiple sclerosis (RRMS) comprising administering to the patient 0.5 ml of an aqueous pharmaceutical solution of 20 mg glatiramer acetate and 20 mg mannitol.
Owner:TEVA PHARMA IND LTD
Reduced Volume Formulation of Glatiramer Acetate and Methods of Administration
InactiveUS20110060279A1Reduce frequencyNervous disorderPeptide/protein ingredientsMANNITOL/SORBITOLHuman patient
A method for reducing frequency of relapses in a human patient afflicted with relapsing-remitting multiple sclerosis (RRMS) comprising administering to the patient 0.5 ml of an aqueous pharmaceutical solution of 20 mg glatiramer acetate and 20 mg mannitol.
Owner:TEVA PHARMA IND LTD
Rituximab induction therapy followed by glatiramer acetate therapy
InactiveUS20140271630A1Organic active ingredientsNervous disorderAutoimmune conditionAutoimmune disease
The present invention provides a method of treating a subject afflicted with a form of multiple sclerosis or presenting a clinically isolated syndrome comprising periodic administration of an amount of rituximab at least twice to the subject followed by periodic administration of an amount of glatiramer acetate to the subject, wherein the amounts are effective to treat the subject. The present invention also provides a method of treating a subject afflicted with an immune disease, comprising periodic administration of an amount of rituximab at least twice to the subject followed by periodic administration of an amount of glatiramer acetate to the subject wherein the amounts are effective to treat the subject, and wherein the immune disease is an autoimmune disease, an arthritic condition, a demyelinating disease, an inflammatory disease, multiple sclerosis, relapsing-remitting multiple sclerosis, diabetes mellitus, psoriasis, rheumatoid arthritis, inflammatory bowel disease, Crohn's disease, or systemic lupus erythematosus.
Owner:TEVA PHARMA IND LTD
Biomarkers predictive of therapeutic responsiveness to ifnb and uses thereof
InactiveUS20120328567A1Effective monitoringEffectively treat multiple sclerosisNervous disorderPeptide/protein ingredientsRelapsing remittingTherapy response
Methods, assays and kits for the identification, assessment and / or treatment of a subject having multiple sclerosis (MS) (e.g., a patient with relapsing-remitting multiple sclerosis (RRMS)) are disclosed.
Owner:BIOGEN MA INC
Treatment of multiple sclerosis with laquinimod
The subject invention provides for methods of reducing the relapse rate and / or reducing the accumulation of physical disability in a relapsing-remitting multiple sclerosis human patient, the method comprising orally administering to the patient a daily dose of 0.6 mg laquinimod.The subject invention also provides for pharmaceutical oral unit dosage forms of 0.6 mg laquinimod for use in reducing the relapse rate and / or for use in reducing the accumulation of physical disability in a relapsing-remitting multiple sclerosis human patient.
Owner:TEVA PHARMA IND LTD
Diagnosis of multiple sclerosis
The present invention relates to methods and kits for diagnosing multiple sclerosis (MS) in a subject. Particularly, the present invention relates to methods and kits for diagnosing a subtype of MS in a subject, the subtype selected from relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), primary progressive MS (PPMS) and a pathologic sub-type of MS lesions selected from Pattern I and Pattern II MS lesion.
Owner:YEDA RES & DEV CO LTD +1
Low frequency glatiramer acetate therapy
InactiveCN102625657AIncreased GA treatment toleranceBiocideNervous disorderHypodermoclysisSubcutaneous injection
A method of alleviating a symptom of relapsing-remitting multiple sclerosis in a human patient suffering from relapsing-remitting multiple sclerosis or a patient who has experienced a first clinical episode and is determined to be at high risk of developing clinically definite multiple sclerosis comprising administering to the human patient three subcutaneous injections of a therapeutically effective dose of glatiramer acetate over a period of seven days with at least one day between every subcutaneous injection so as to thereby alleviate the symptom of the patient.
Owner:YEDA RES & DEV CO LTD
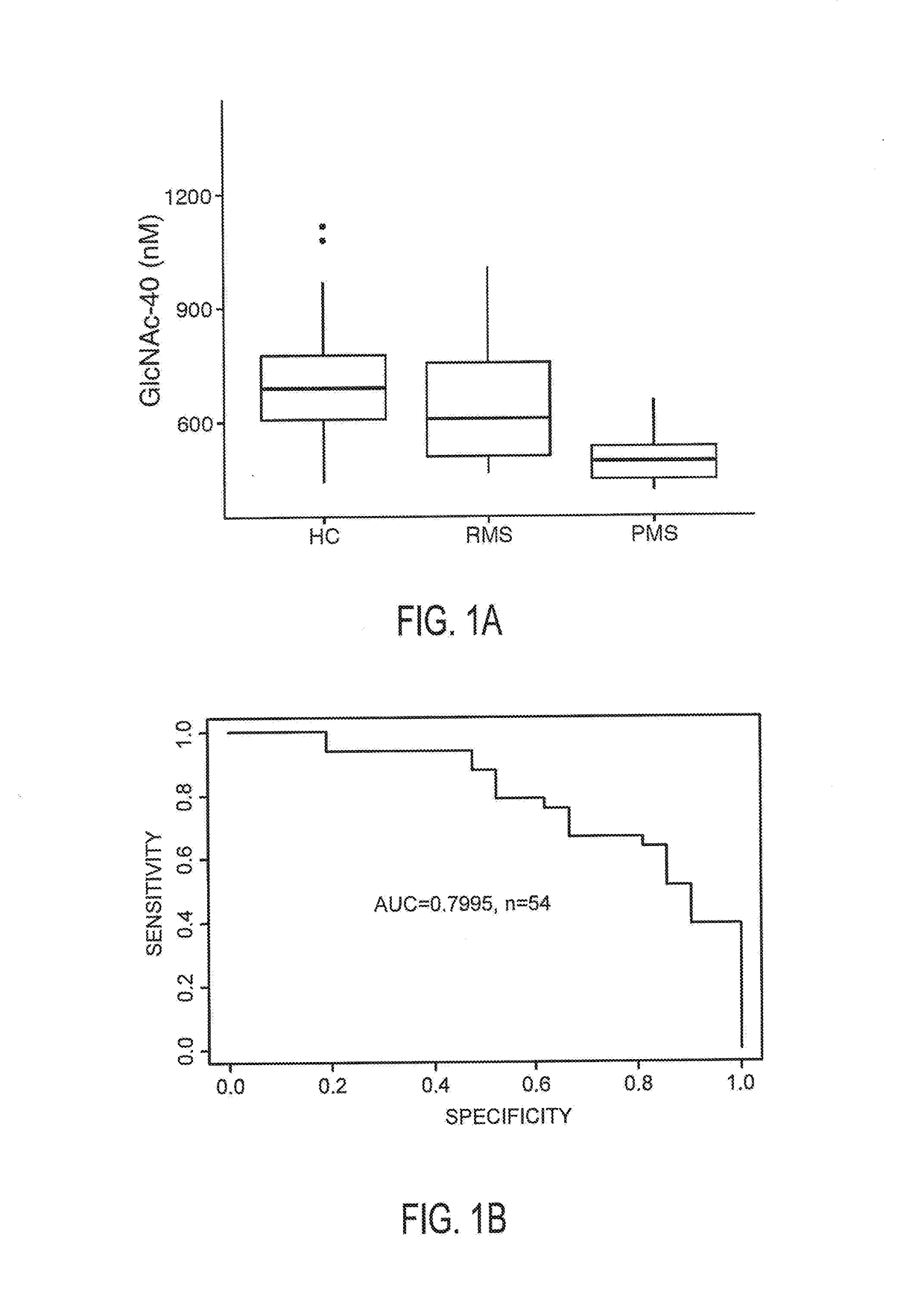
N-Acetyl Glucosamine as a Biomarker of MS Disease Course
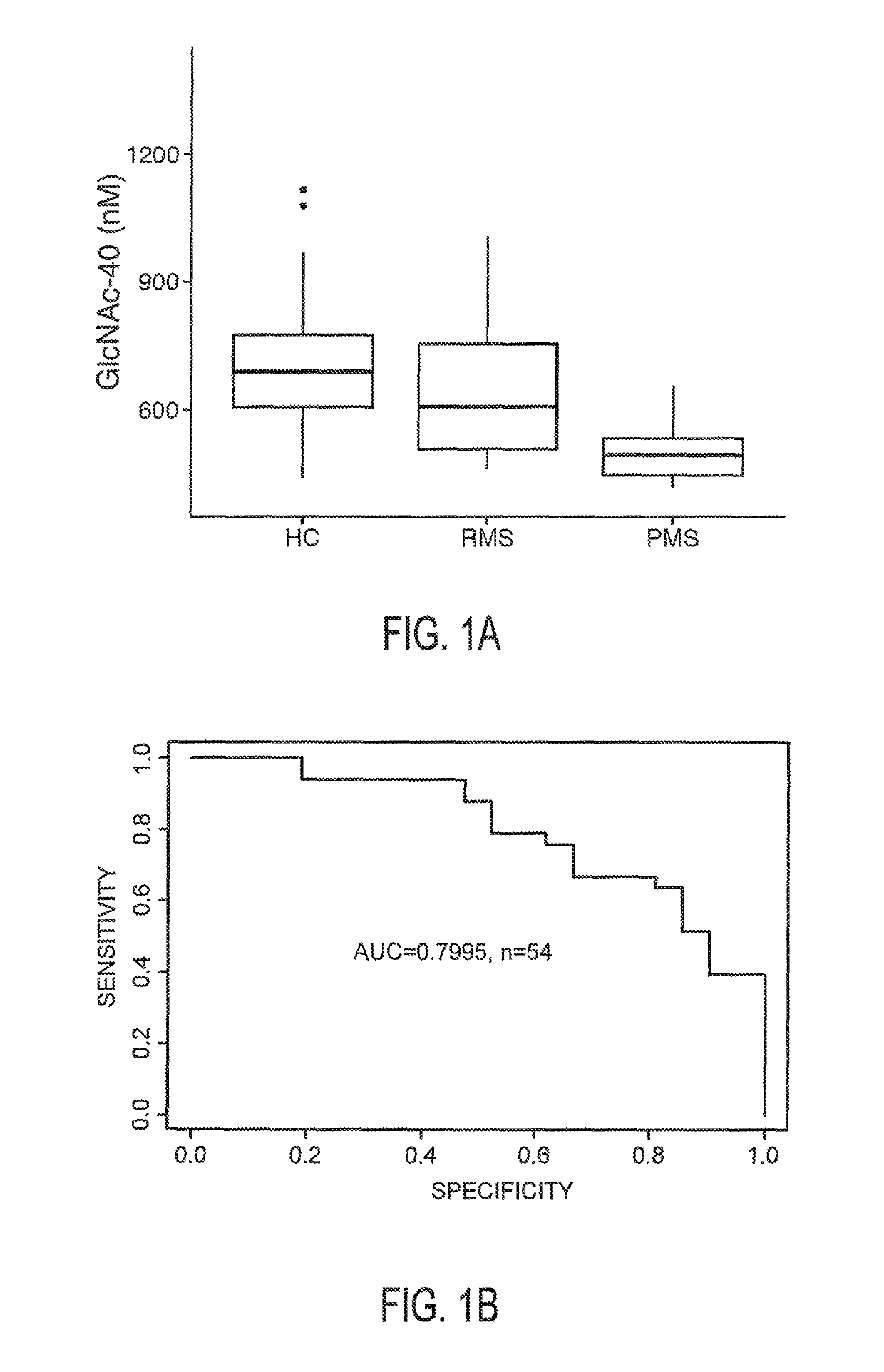
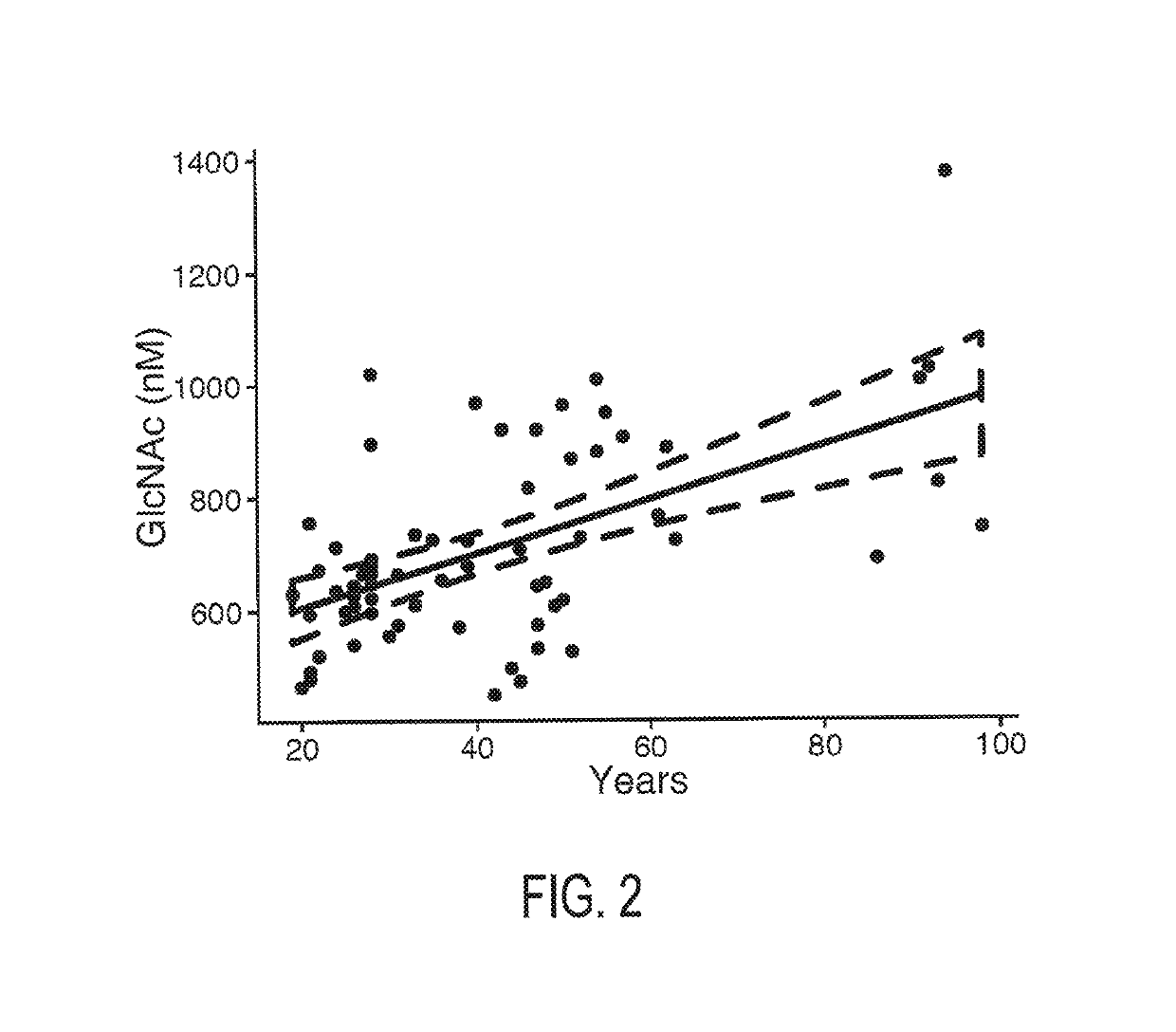
The invention provides the art with a powerful diagnostic method of distinguishing relapse-remitting MS subjects from progressive MS subjects, based on the measurement of serum concentrations of N-acetylglucosamine (GlcNAc,), for the first time enabling rapid diagnosis of the progressive form of MS. GlcNAc serum concentration can also be used to assess neurodegenerative status and MS progression in subjects suffering from MS or other neurological conditions. The methods of the invention also allow for the identification of new therapeutics for MS and other neurological conditions and also enables the personalized efficacy assessment of a potential therapy for an MS subject.
Owner:CHARITE UNIVS MEDIZIN BERLIN +2
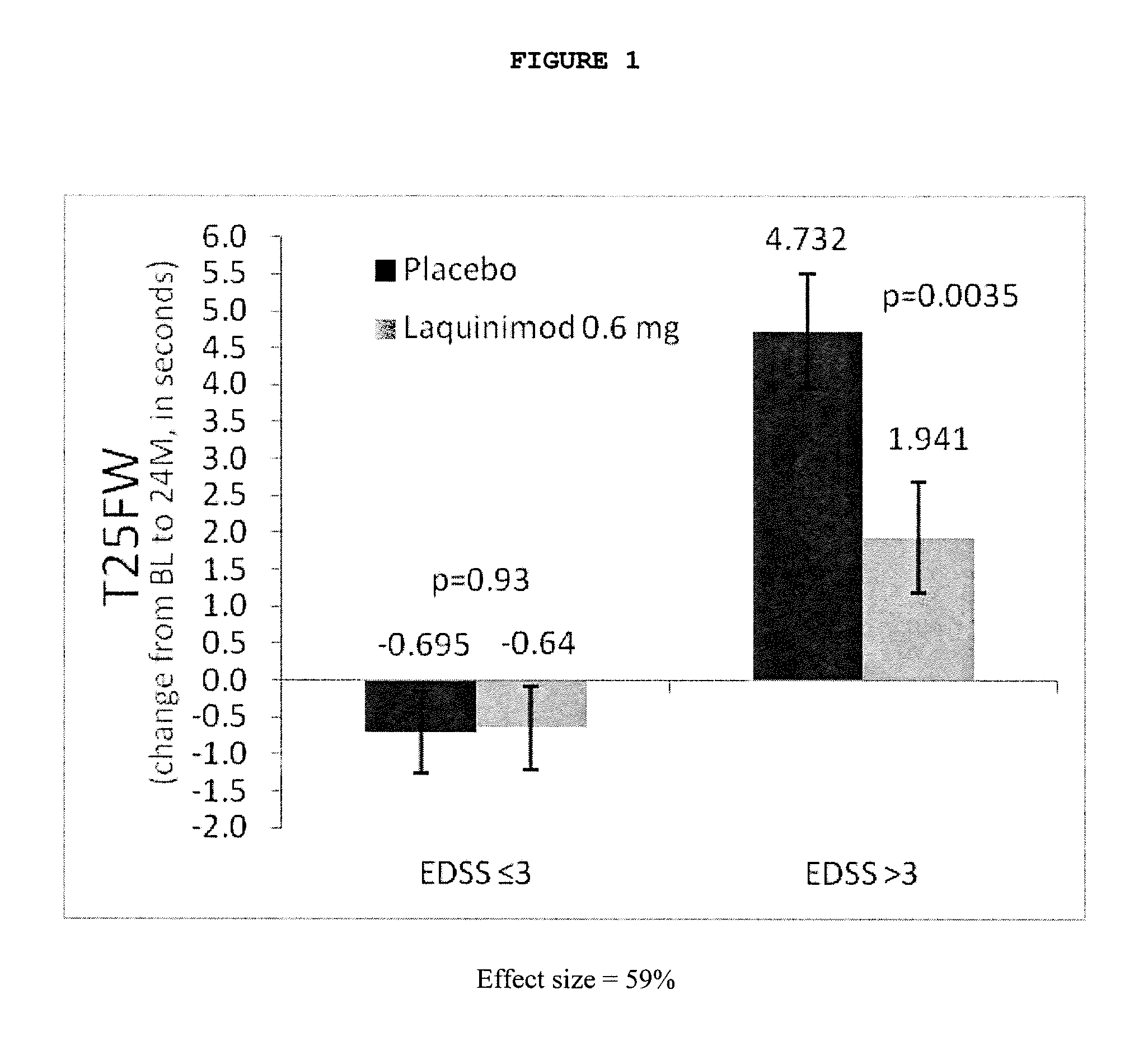
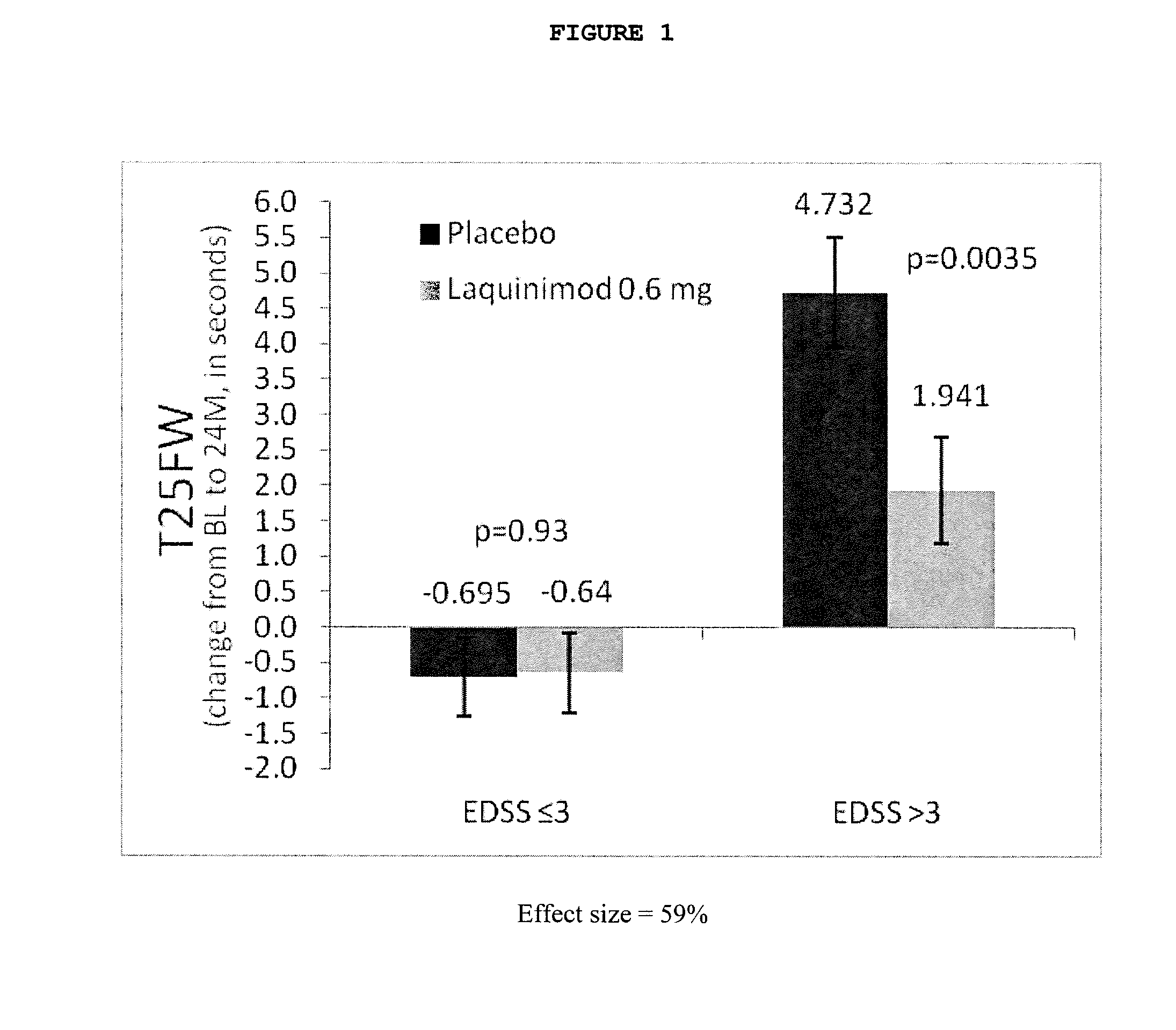
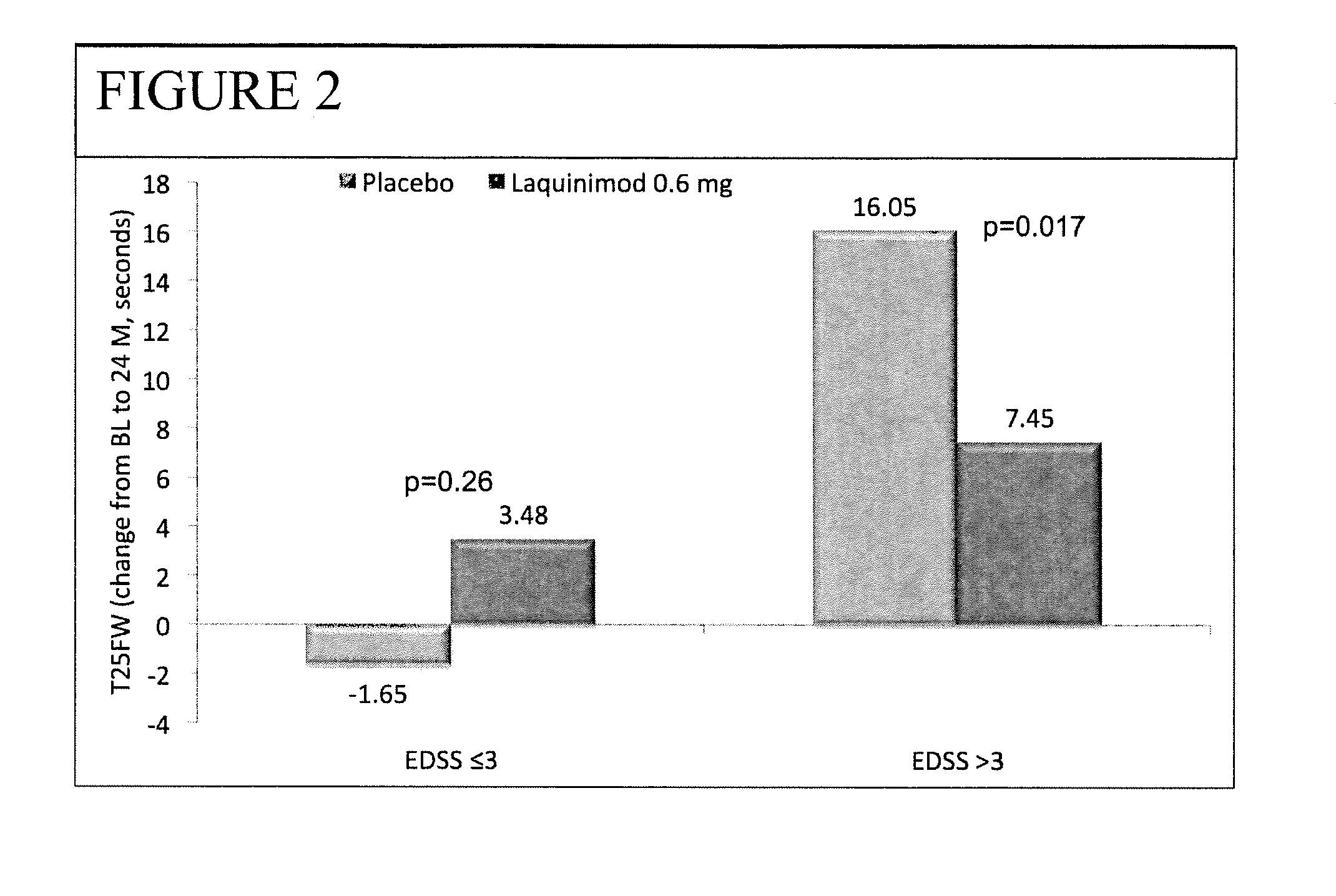
Laquinimod for the treatment of relapsing-remitting multiple sclerosis (RRMS) patients with a high disability status
InactiveUS20150306088A1Reducing ambulatory deteriorationReduce ambulatory deteriorationBiocideNervous disorderLaquinimodHuman patient
This invention provides a method for treating or for reducing ambulatory deterioration in a human patient diagnosed to be afflicted with relapsing-remitting multiple sclerosis (RRMS) and having a high baseline disability score according to the Kurtzke Expanded Disability Status Scale (EDSS), comprising periodically administering to only the patient diagnosed with RRMS and having a high baseline disability score an amount of laquinimod effective to treat the patient or to reduce ambulatory deterioration. This invention further provides pharmaceutical compositions and packages comprising an effective amount of laquinimod for treating a human patient diagnosed to be afflicted with RRMS and having a high baseline disability score according to the EDSS.
Owner:TEVA PHARMA IND LTD
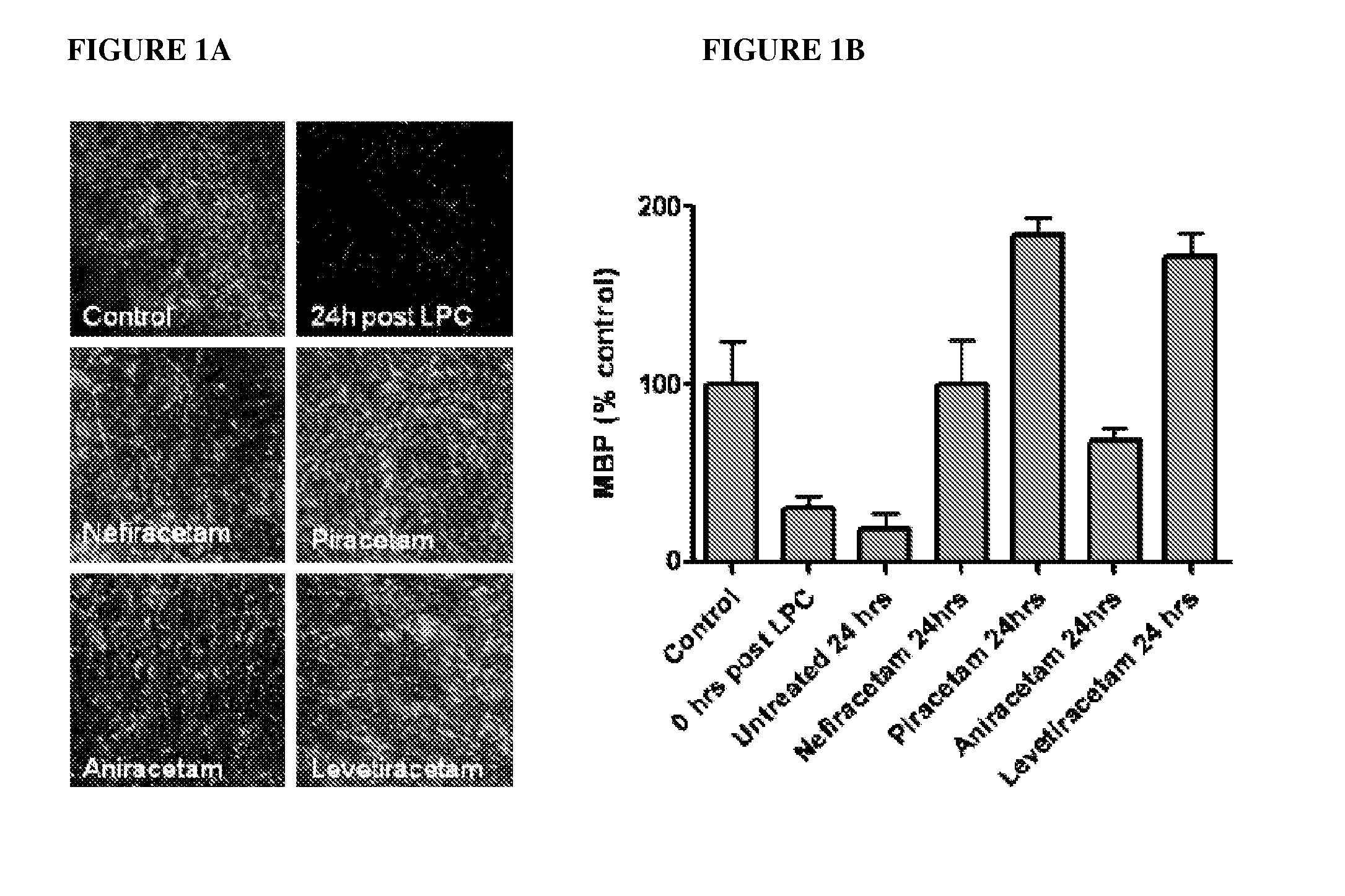
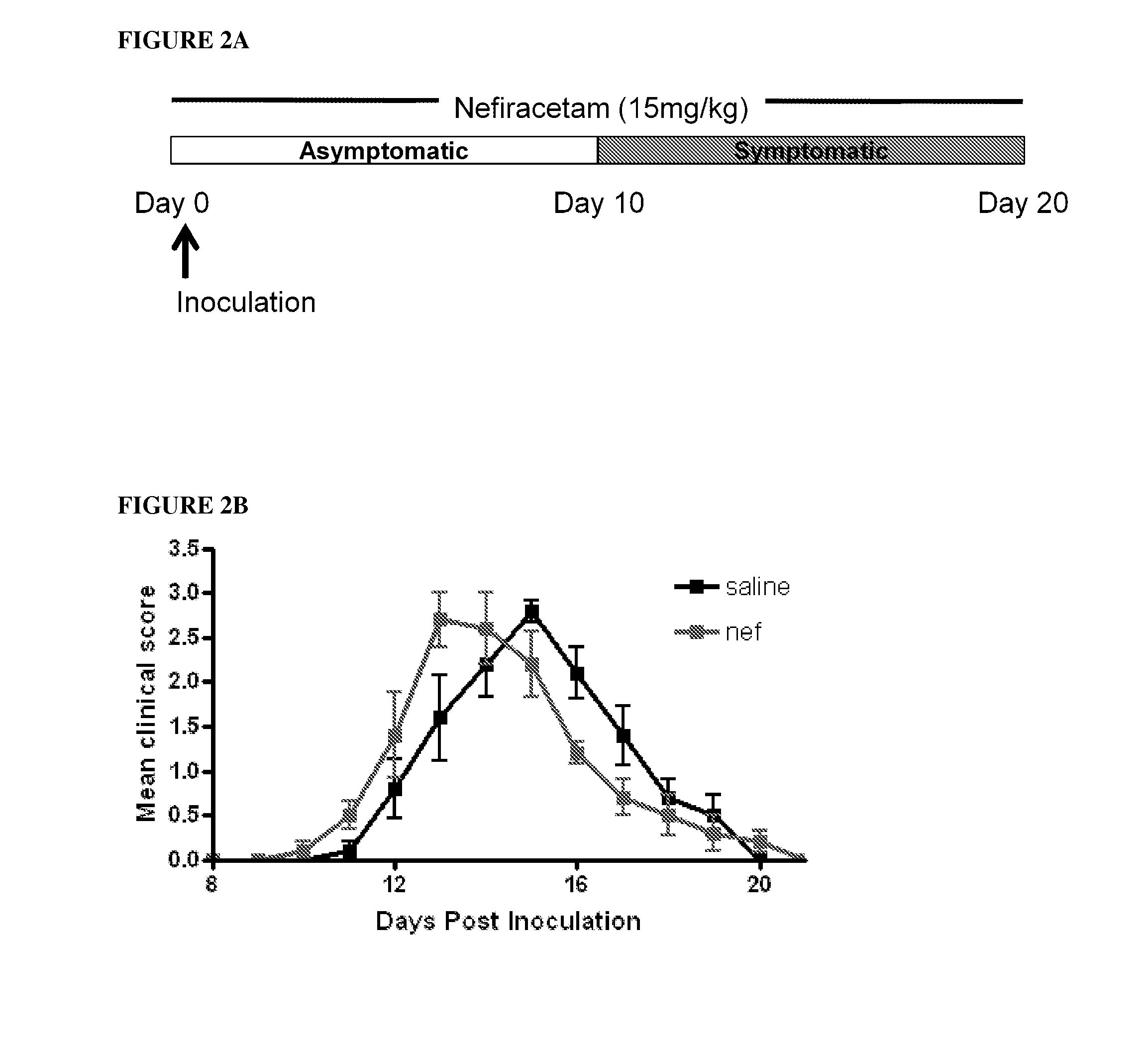
Causal therapy of diseases or conditions associated with CNS or pns demyelination
ActiveUS20120269762A1Maintain integrityAccelerates myelin repairBiocideNervous disorderActive agentRemyelination
The invention broadly relates to the use of the Active in the causal treatment of a disease caused by axonal demyelination, in which the Active maintains the integrity of myelination (for example by promoting remyelination, and / or preventing demyelination, of the axonal sheaths). The invention is particularly directed to the causal treatment of CNS demyelination diseases, for example MS, especially primary progressive MS and / or relapse remitting MS, and PNS demyelination diseases, for example Charcot-Marie-Tooth Disease. The Active of the invention may be suitably administered when a patient is in relapse (i.e. upon relapse), and be continued while the patient is in relapse, with a view to attenuating the severity of the relapse, and / or accelerating disease remission. Alternatively, the Active may be administered continuously with a view to prolonging the remission period, and / or attenuating the severity of the relapse, and / or preventing relapse. The invention also relates to the use of the Active as a treatment for symptoms of demyelination disease, especially MS, selected from vision deficits, motor control deficits, and sensation deficits.
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
Laquinimod for the treatment of relapsing-remitting multiple sclerosis (RRMS) patients with a high disability status
InactiveUS9662322B2Reducing ambulatory deteriorationHigh baseline disability scoreNervous disorderPeptide/protein ingredientsLaquinimodHuman patient
This invention provides a method for treating or for reducing ambulatory deterioration in a human patient diagnosed to be afflicted with relapsing-remitting multiple sclerosis (RRMS) and having a high baseline disability score according to the Kurtzke Expanded Disability Status Scale (EDSS), comprising periodically administering to only the patient diagnosed with RRMS and having a high baseline disability score an amount of laquinimod effective to treat the patient or to reduce ambulatory deterioration. This invention further provides pharmaceutical compositions and packages comprising an effective amount of laquinimod for treating a human patient diagnosed to be afflicted with RRMS and having a high baseline disability score according to the EDSS.
Owner:TEVA PHARMA IND LTD
Biomarkers predictive of therapeutic responsiveness to ifnb and uses thereof
InactiveUS20160045570A1Evaluate responsivenessAvoid exacerbationNervous disorderPeptide/protein ingredientsBiomarker (petroleum)Treatment response
Methods, assays and kits for the identification, assessment and / or treatment of a subject having multiple sclerosis (MS) (e.g., a patient with relapsing-remitting multiple sclerosis (RRMS)) are disclosed.
Owner:BIOGEN MA INC
Diagnosis and prognosis of multiple sclerosis
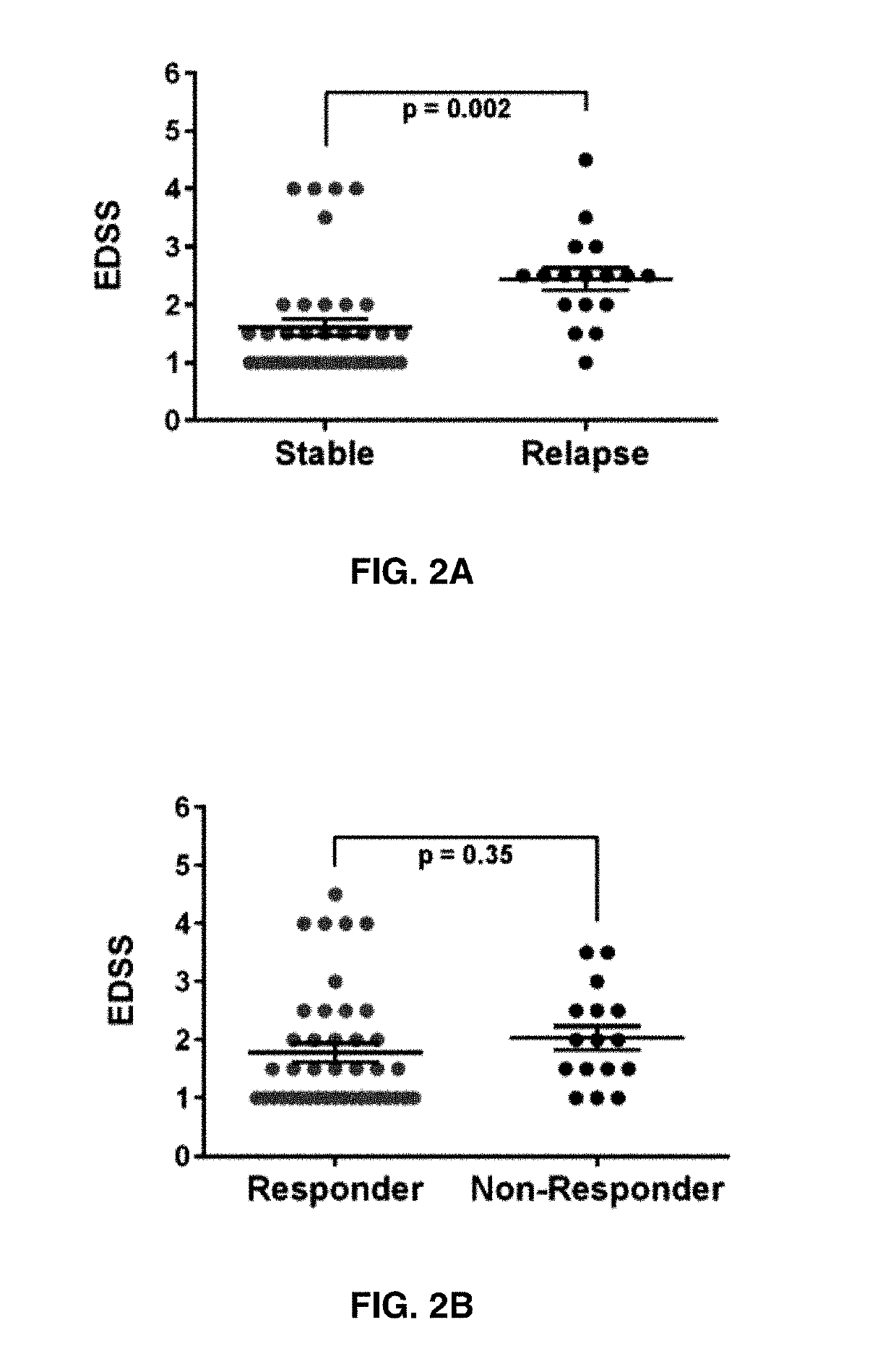
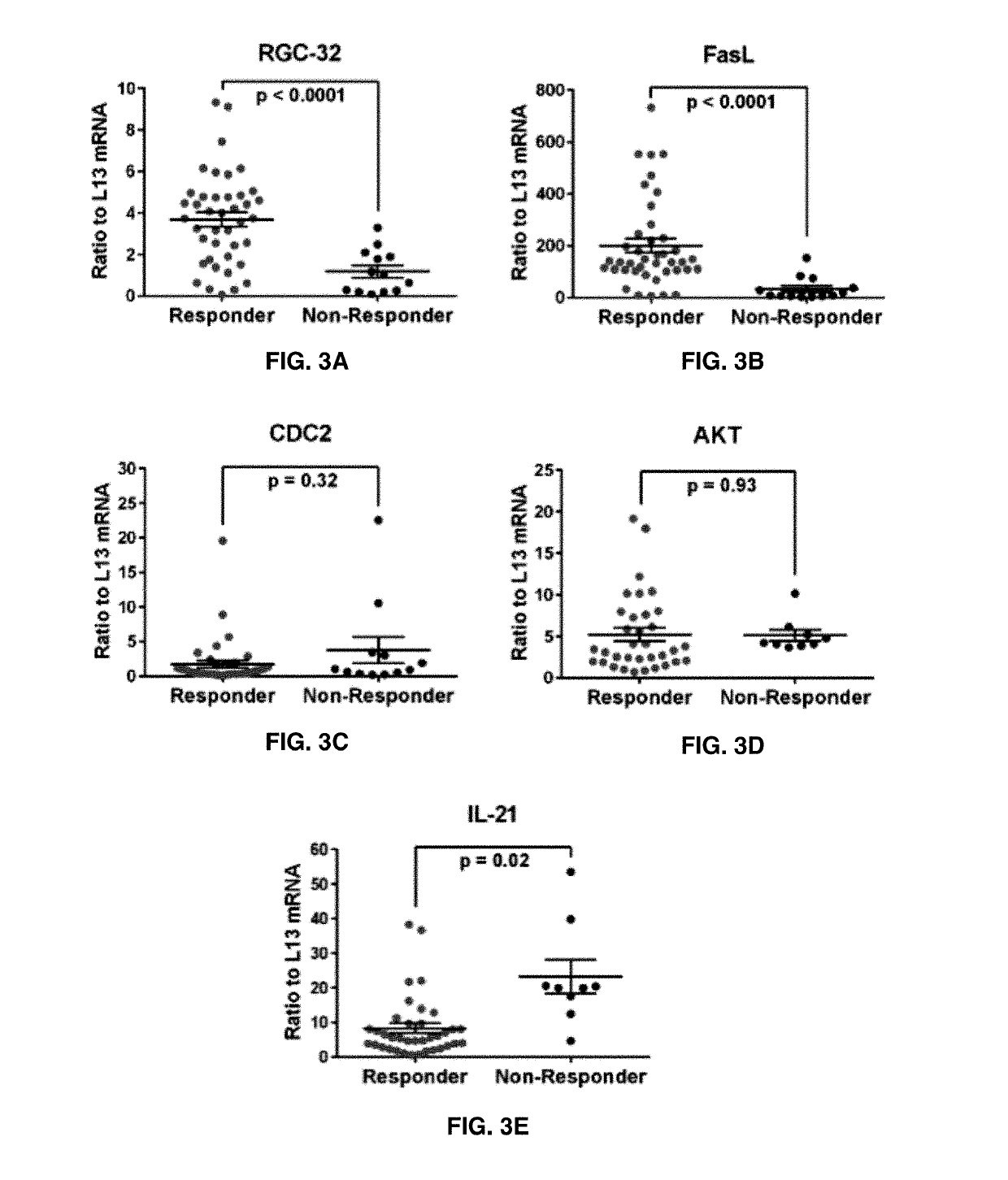
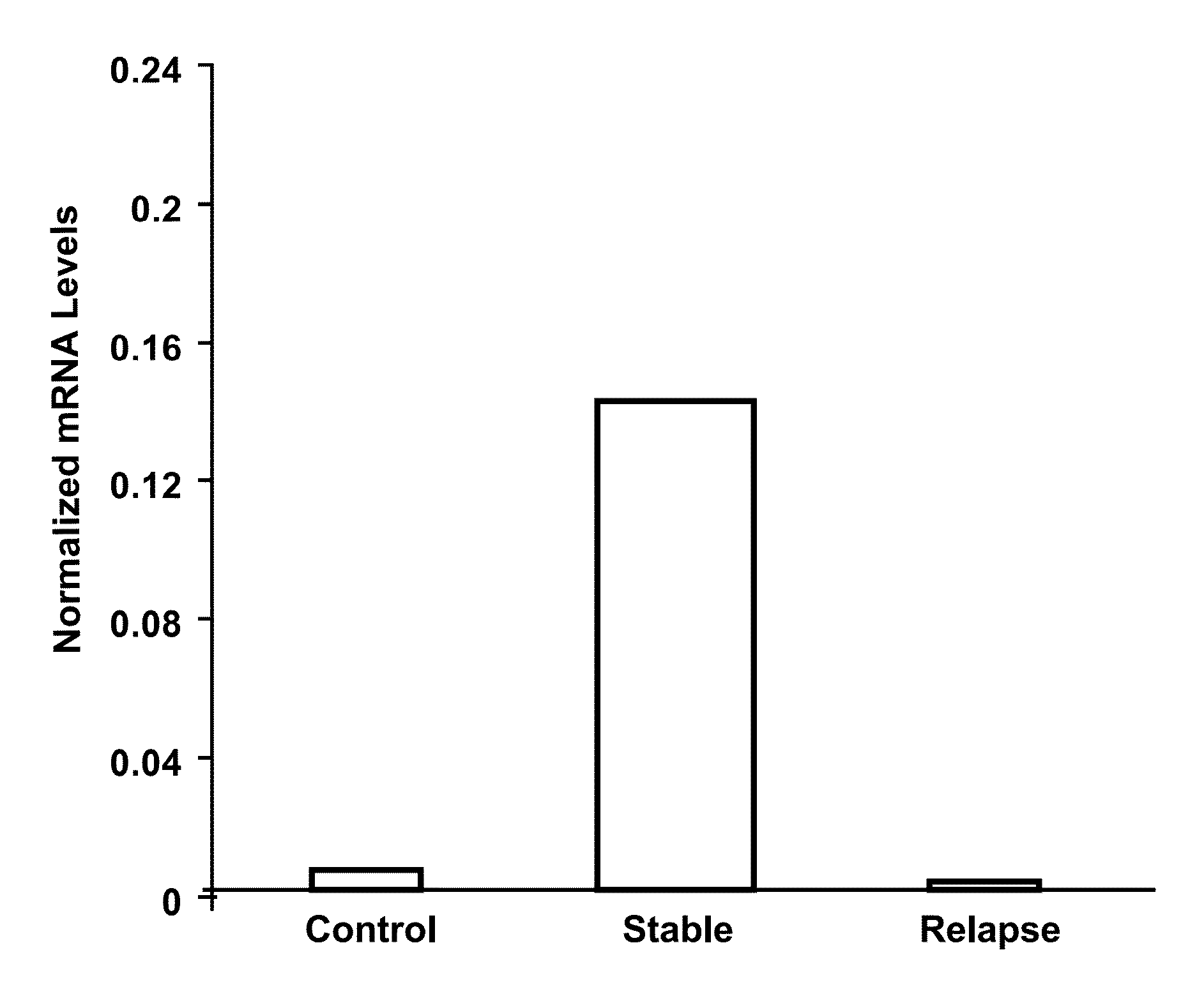
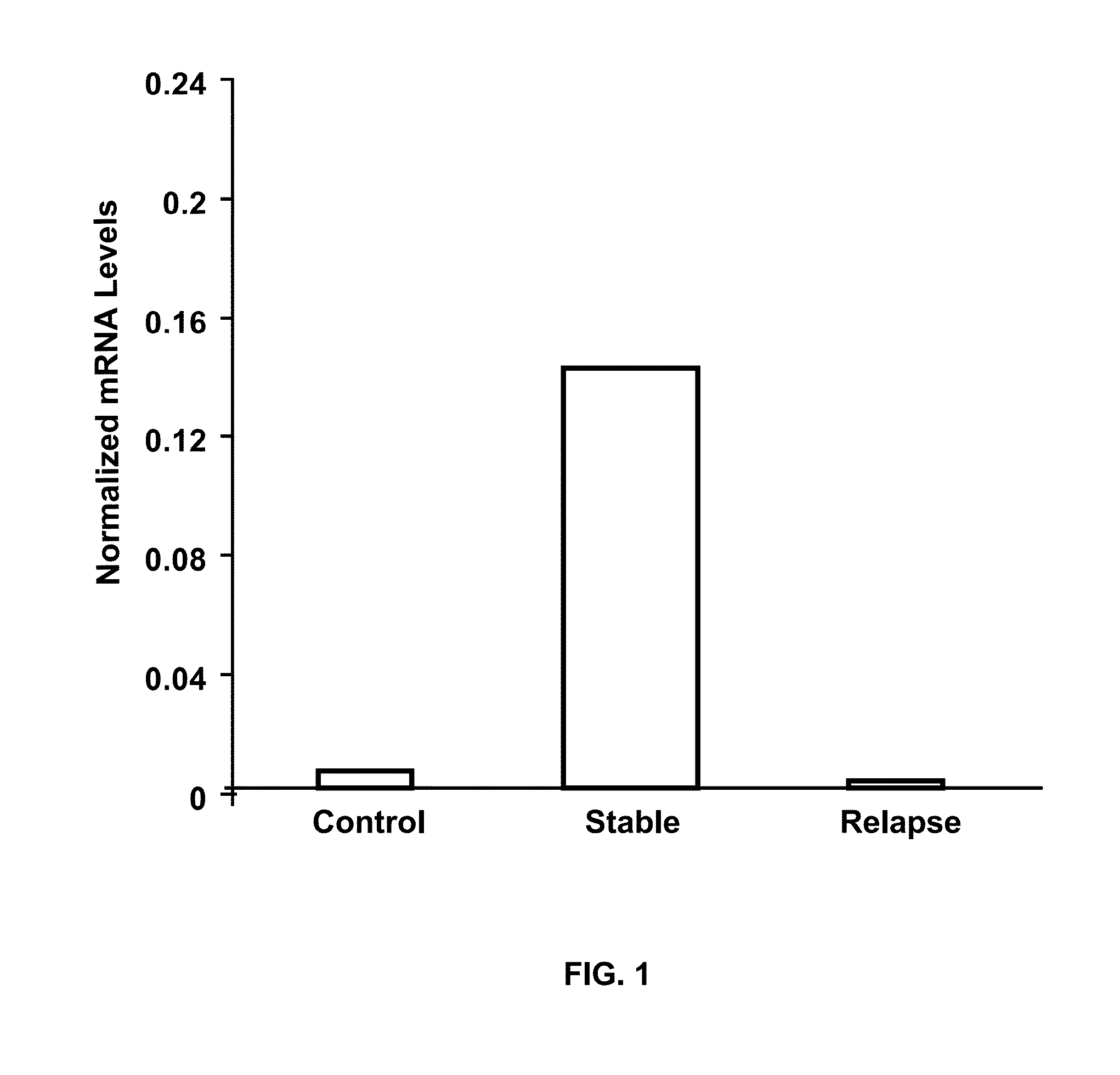
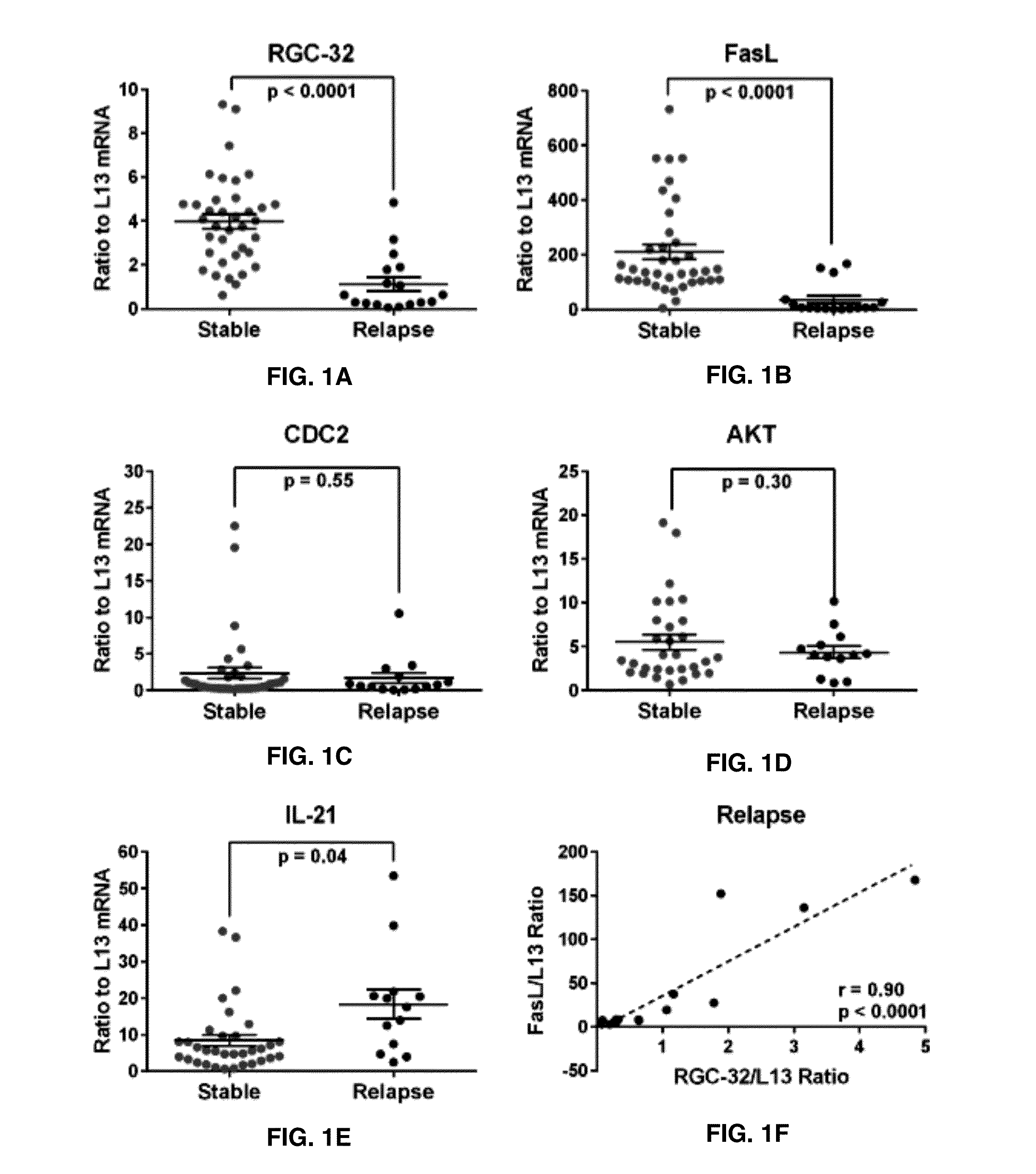
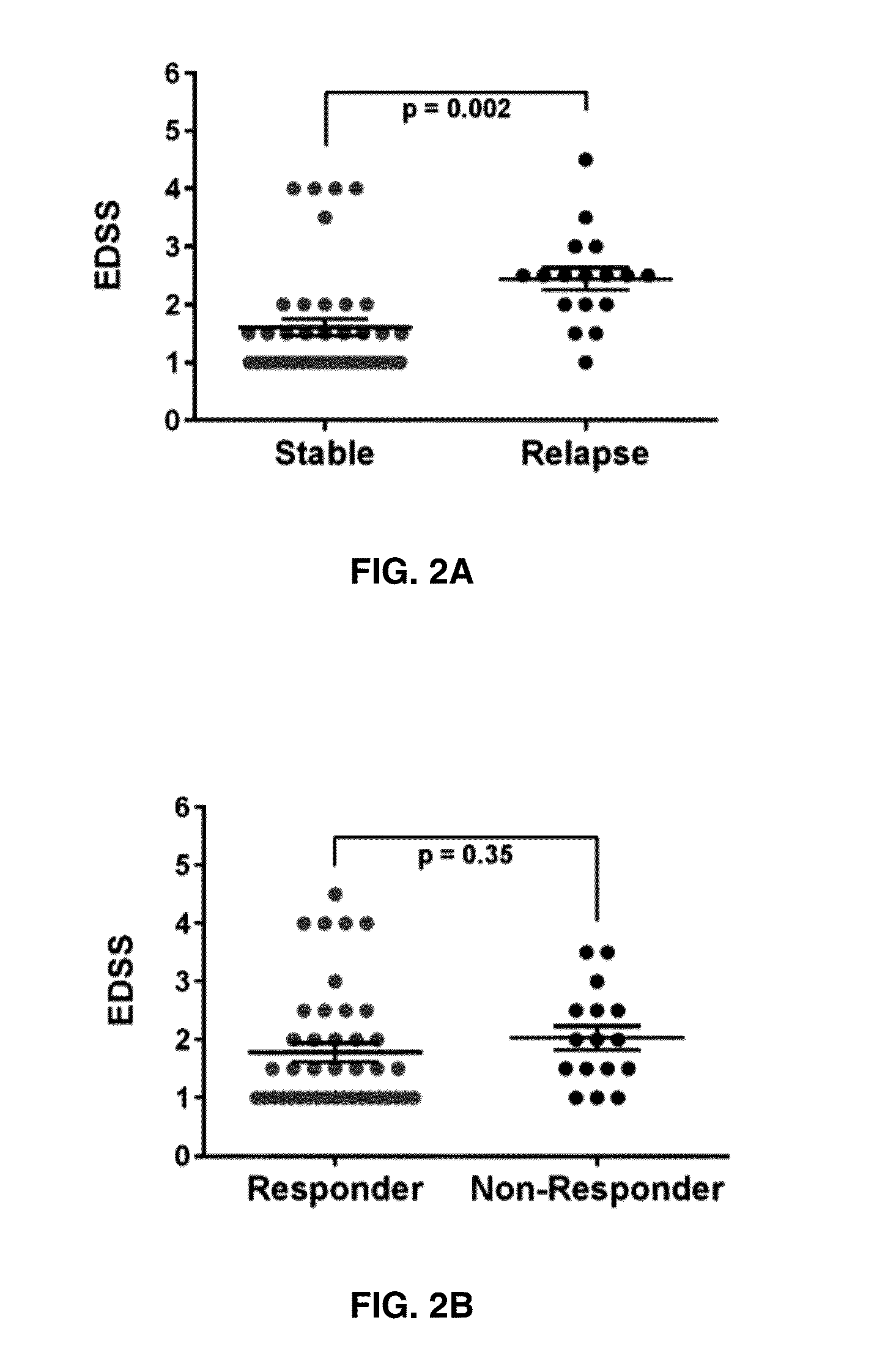
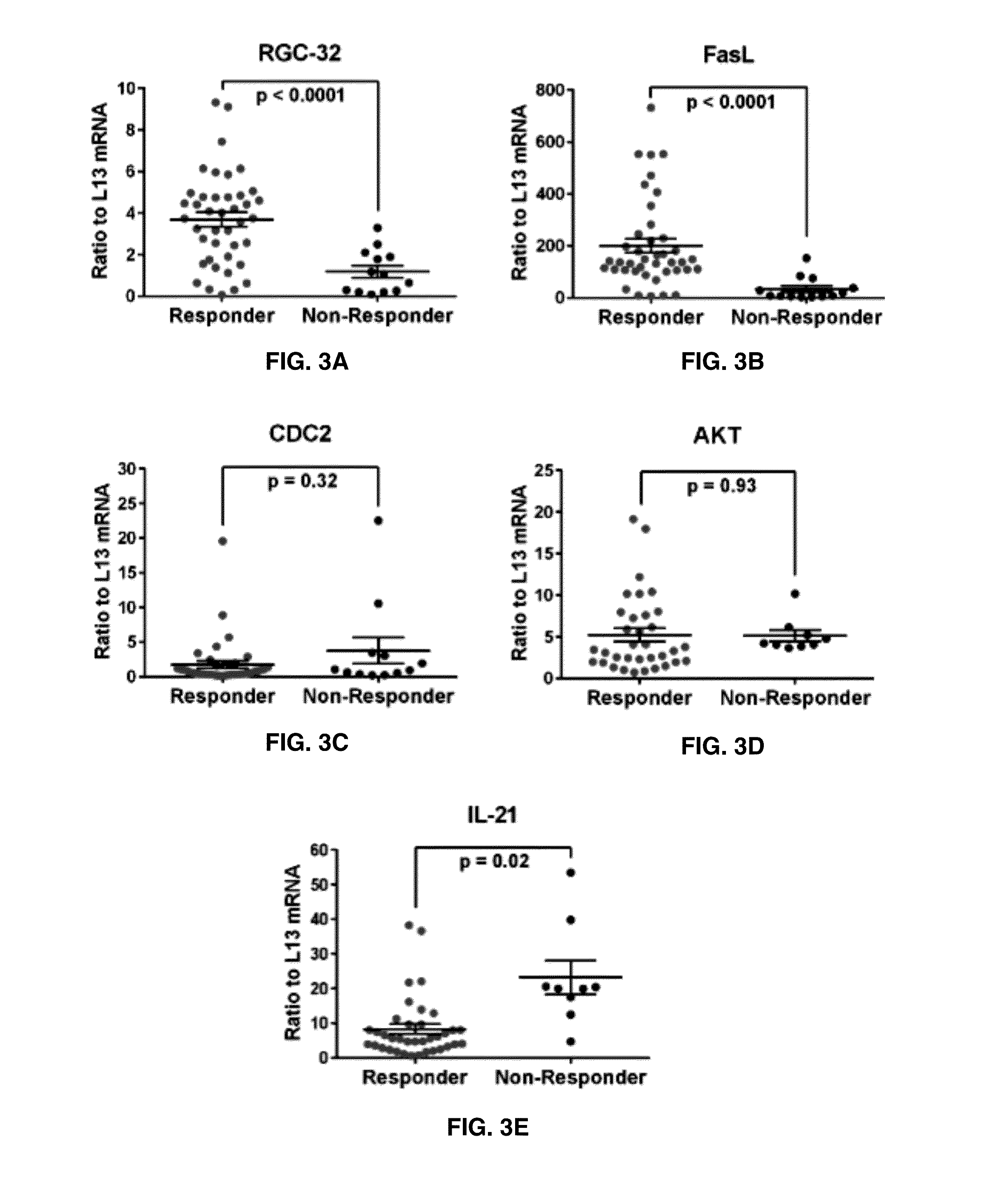
The present invention provides a method for determining whether an individual with relapsing-remitting multiple sclerosis will suffer a relapse or respond to treatment for MS. A ratio of mRNA levels of Response Gene to Complement-32, FasL or IL-21 to L13 determined for an individual provides a normalized level which is compared to a cut-off value. A normalized level of Response Gene to Complement-32 greater than 2.52, a normalized level of FasL greater than 85.4 and a normalized level of IL-21 less than 11.9, respectively, indicates the individual will have or is having a relapse of multiple sclerosis. Also provided are methods for determining whether an individual will respond positively or is responding positively to glatiramer treatment and whether the individual is in a period of stable disease or is not at risk for relapse of multiple sclerosis by comparing normalized levels with the respective cut-off levels.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
In-vitro methods for the detection of autoimmune diseases or conditions
ActiveUS11447825B2Increased phosphorylationHigh expressionMicrobiological testing/measurementBiological material analysisAutoimmune conditionAutoimmune disease
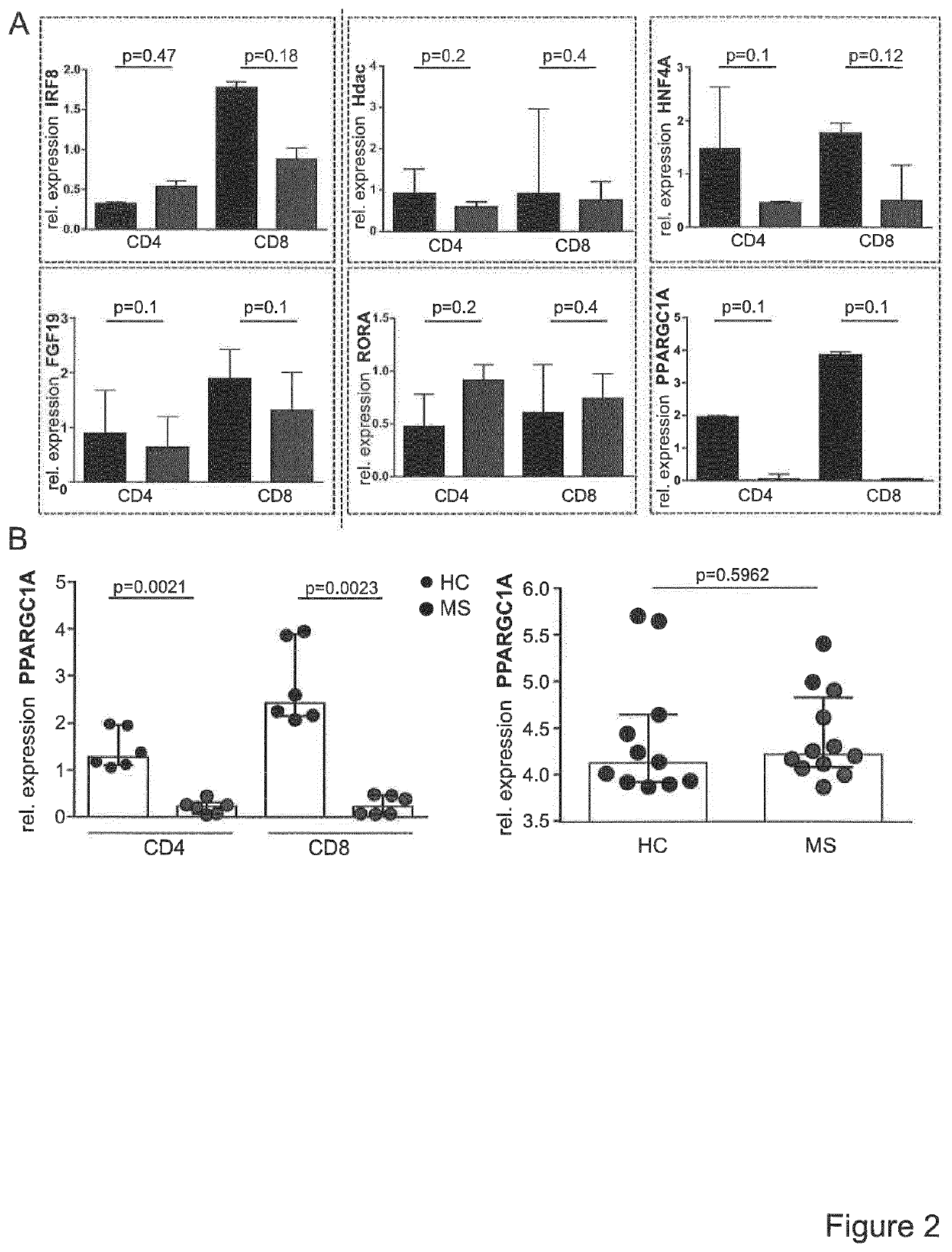
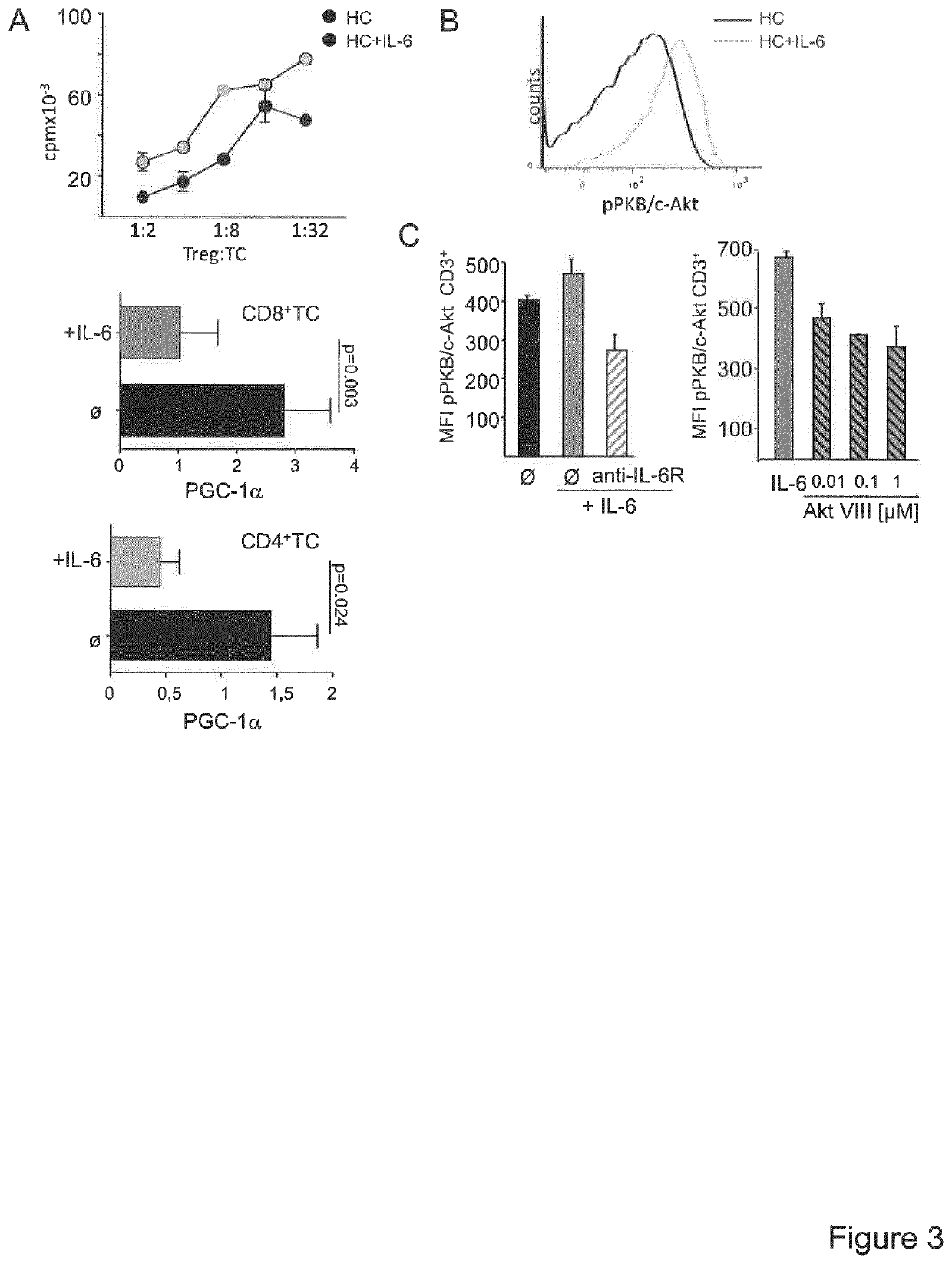
A method for the detection of impaired responsiveness of CD4+ T-cells to regulatory T-cells (Treg), Treg resistance, by measuring the expression levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, PPARGC1A (PGC-1α) in activated CD4+ T-cells, in particular in patients suffering from relapsing remitting multiple sclerosis. The invention relates to an in vitro screening method for the detection of an autoimmune disease or a condition, comprising the steps of generating a functional gene expression profile by measuring the expression levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, PPARGC1A (PGC-1α) in Treg-resistant CD4+ T-cells from patients suffering of an autoimmune disease or condition, and comparing the obtained gene expression profile with the expression profile from Treg-sensitive CD4+ T-cells from healthy controls. PCG-1α or an upstream regulator of Treg-resistant T-cells HNF4A, Hdac, RORA, ESRRA, LPIN1 can be used in a screening system for the detection of impaired responsiveness of CD4+ T-cells to Treg.
Owner:UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ
Assay and method for predicting therapeutic efficacy of immunoglobulin therapy in individual patients with relapsing remitting multiple sclerosis (RR-MS)
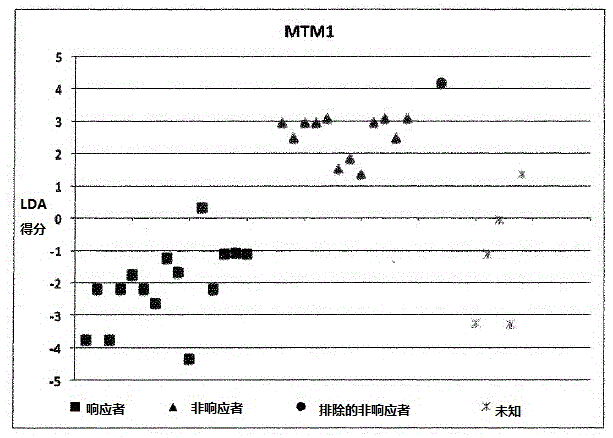
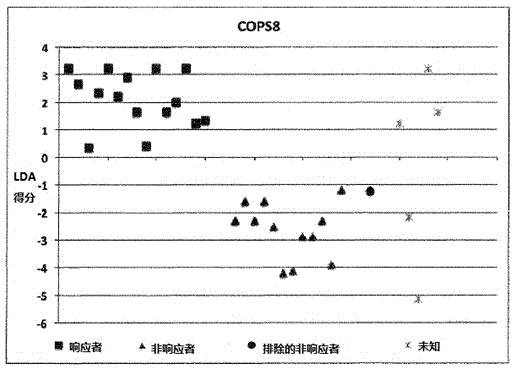
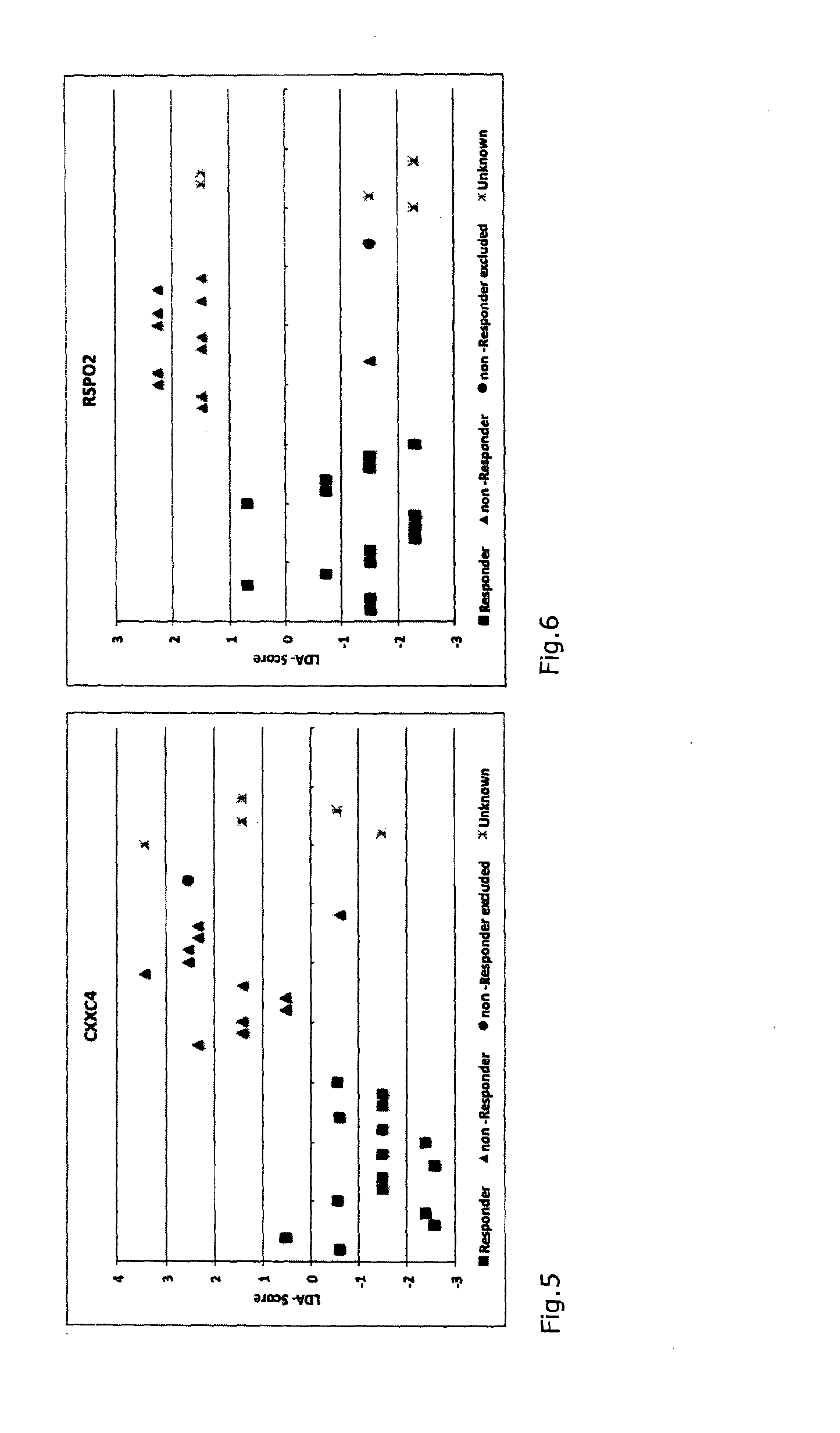
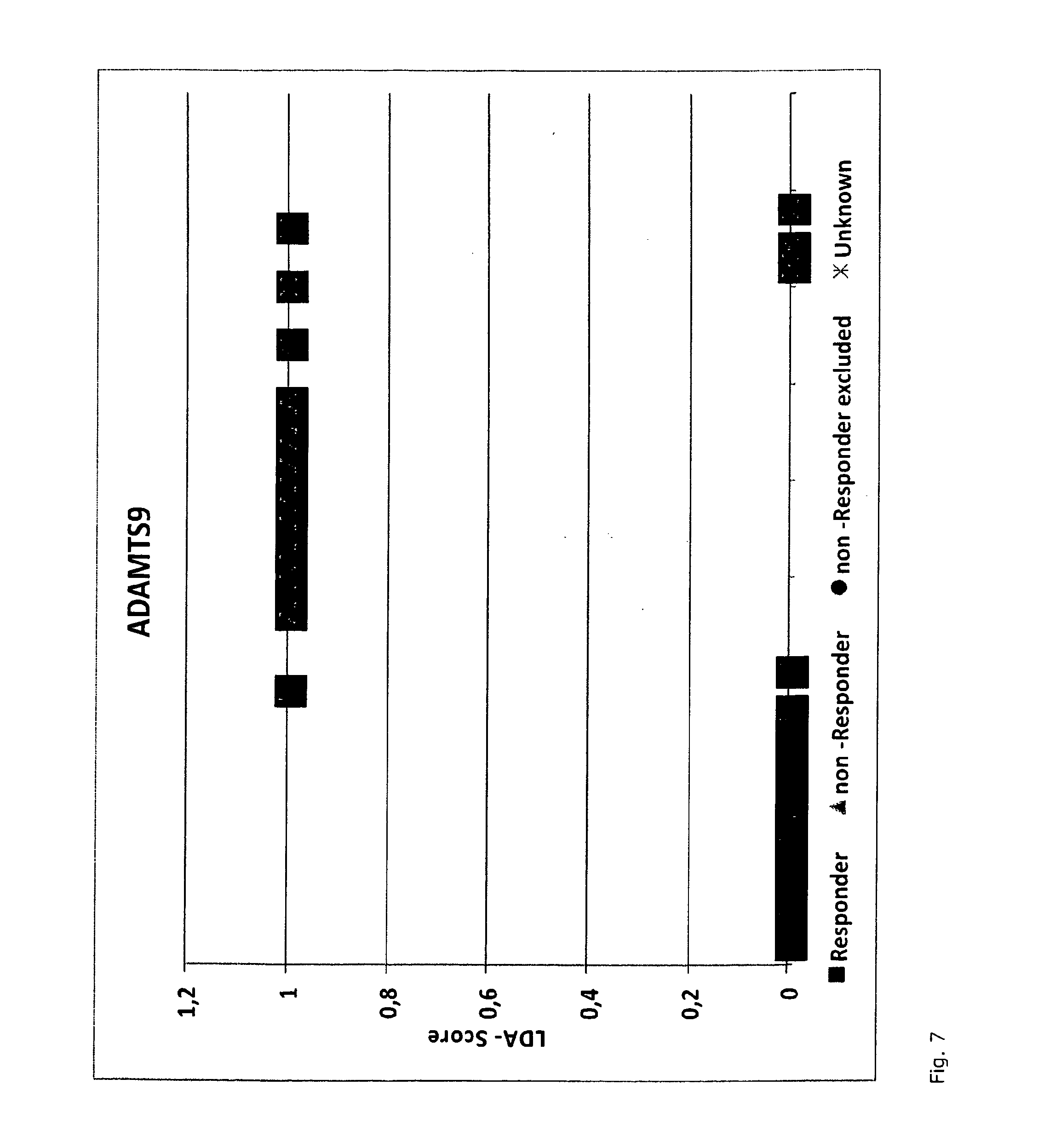
A method for generating a reference for determining the likelihood of response of a patient, suffering from a disease, towards immunoglobulin therapy comprising the steps of a) providing samples of a sufficient number of individuals, in particular at least 10 individuals, the samples containing B- and T-lymphocytes, natural killer cells, invariant T-cells and monocytes of the individuals; b) determination of values of immune parameters which are either static, such as leukocyte subpopulations and cytokine-level in the plasma or functional, like gene expression and cytokine release after lipopolysaccharide (LPS) and / or IVIG stimulation ex vivo; c) the determined values derived from immune parameters of the samples of the individuals are ordered in quartiles and the values belonging to the 1. quartile, values distributed at the low end of the corresponding parameter, are set to “−1”, whereas values distributed in the 4. quartile are set to “+1” for an individual LDA-score calculation and values in between are set to “0”; d) genotyping of at least two of the polynucleotides selected from the group consisting of MTM1, EIF3E, COPS8, ADAMTSL1, CXXC4, RSPO2, OR9Q1, ADAMTS9, KLHDC8A and PRDM9, in the samples of the individuals and awarding the value of 0 for specific homozygous SNP combinations (SNP—Single Nucleotide Polymorphism), which indicates that the blood sample stems from a person which will respond to immunoglobulin (IG) treatment, while awarding the value of 1 for SNP combinations not meeting that criteria, which indicates that the blood sample stems from a person which will not respond to immunoglobulin treatment; e) combining the results of genotyping with results of immuno parameter determination in the calculation of an individual LDA-score (LDA—Linar Discriminant Analysis); f) combining a multiplicity of individual LDA-scores to create a reference Responder Score, which allows discrimination between responders and non-responders. The method can be employed for determining the likelihood of response of a patient, suffering from a disease, towards immunoglobulin therapy.
Owner:OCTAPHARMA +1
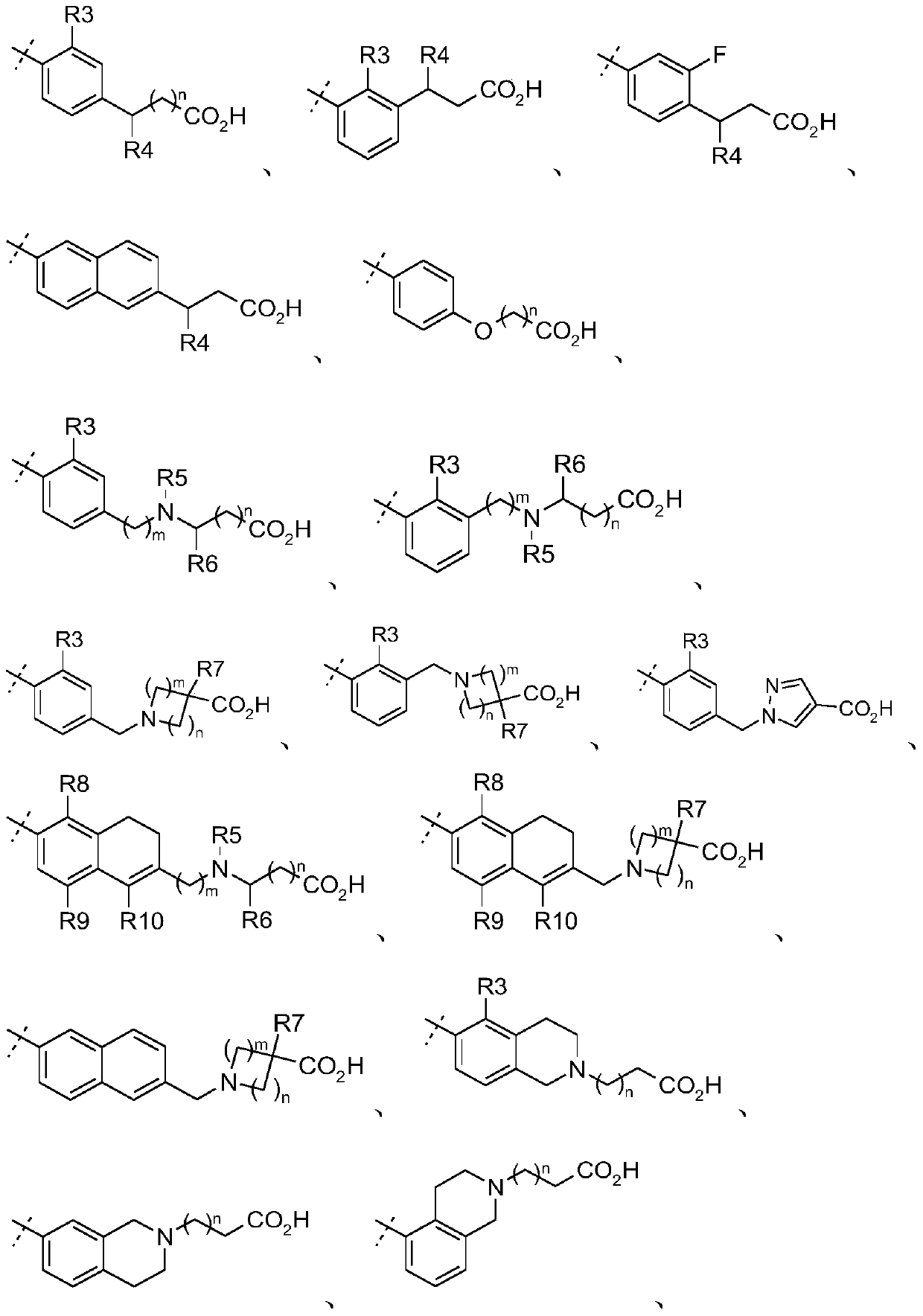
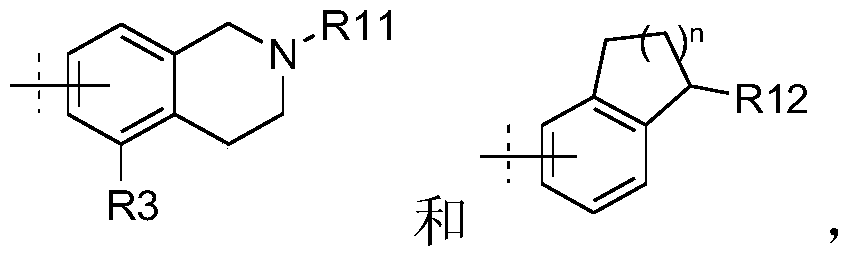
Tri-substituted glycerol compounds for use in the treatment of clinically isolated syndrome and/or multiple sclerosis
InactiveUS20150157651A1Reduce in quantityReduce penetrationBiocideNervous disorderInterferon therapyMedicine
The present invention relates to tri-substituted glycerol compounds according to formula (I) as described herein for use in the treatment of clinically isolated syndrome, relapsing remitting multiple sclerosis (RR-MS) and / or secondary progressive multiple sclerosis (SP-MS). The tri-substituted glycerol compounds according to formula (I) as described herein may also be used for the treatment of multiple sclerosis patients not adequately responding to interferon therapy. The present invention also relates to a tri-substituted glycerol compound as described herein, for use as a medicament, wherein the tri-substituted glycerol compound is administered in combination with at least one further pharmaceutically active compound.
Owner:ALPHAPTOSE +1
Cladribine regimen for treating multiple sclerosis
InactiveCN101090726AWeakening of pathological processesReduced pathological processOrganic active ingredientsNervous disorderRegimenPharmaceutical formulation
The present invention is related to the use of Cladribine for the preparation of a pharmaceutical formulation for the treatment of multiple sclerosis, especially relapsing-remitting multiple sclerosis or early secondary progressive multiple sclerosis, wherein the preparation is to be the orally administered and wherein re-treatments are possible.
Owner:MERCK SERONO SA
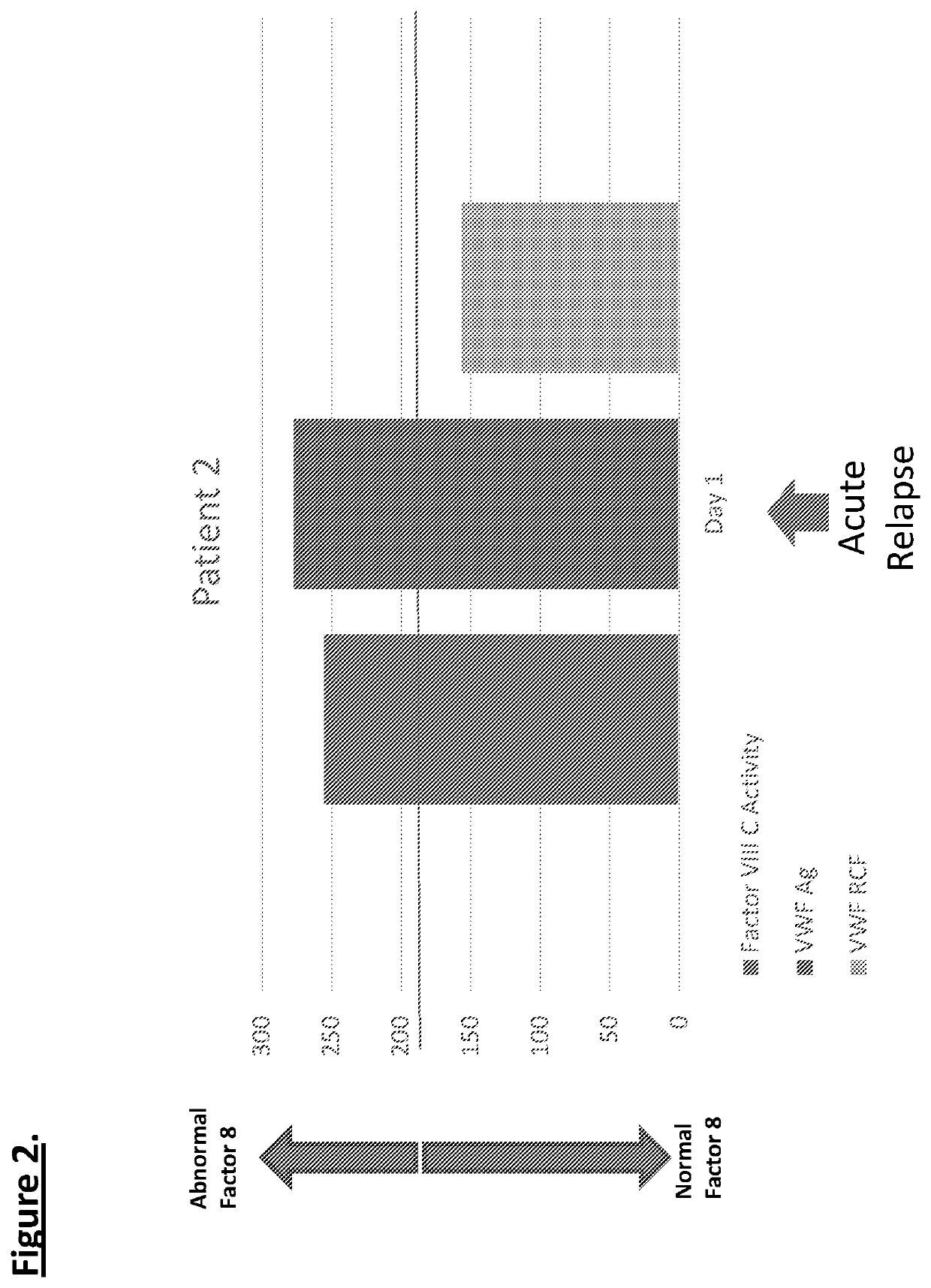
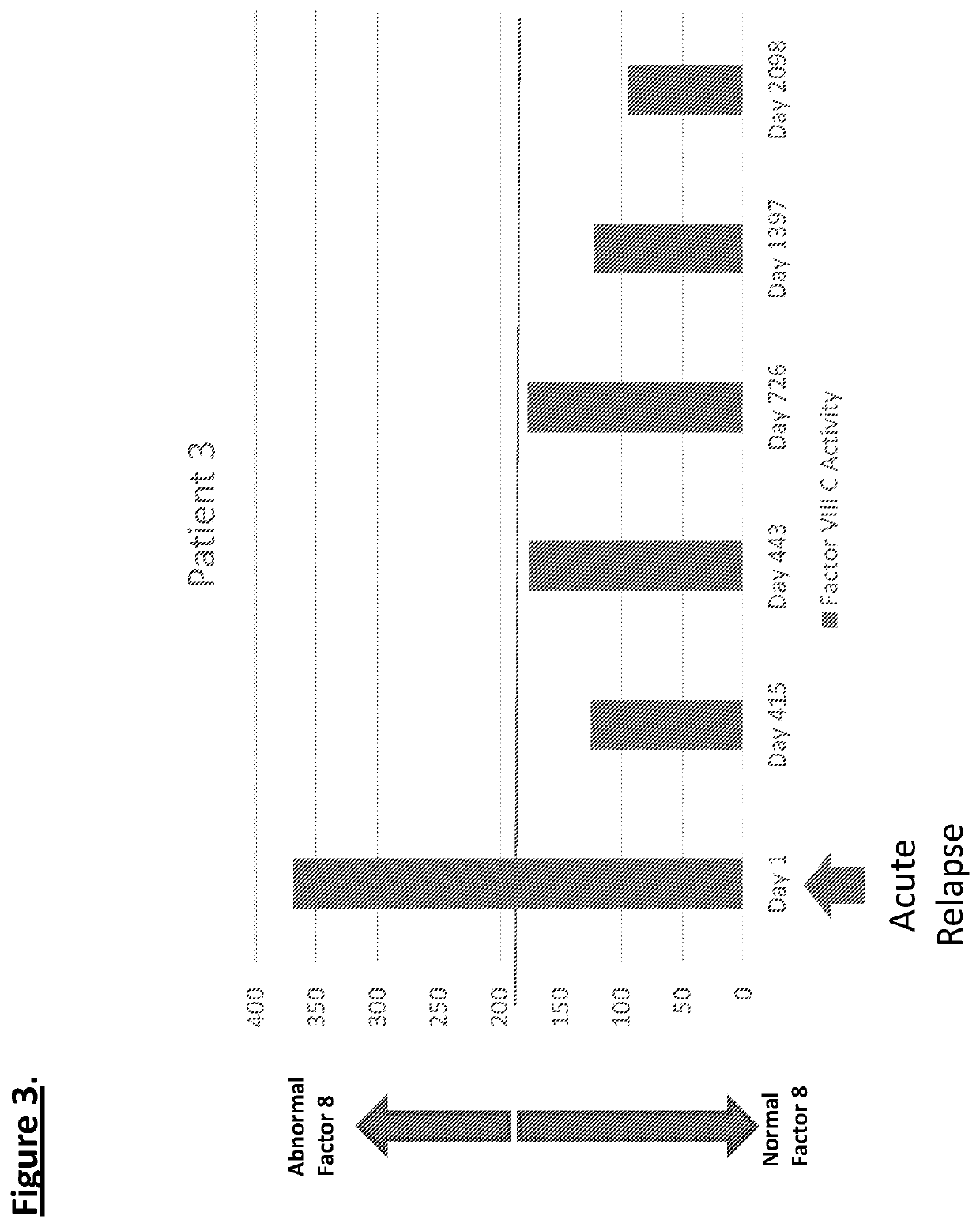
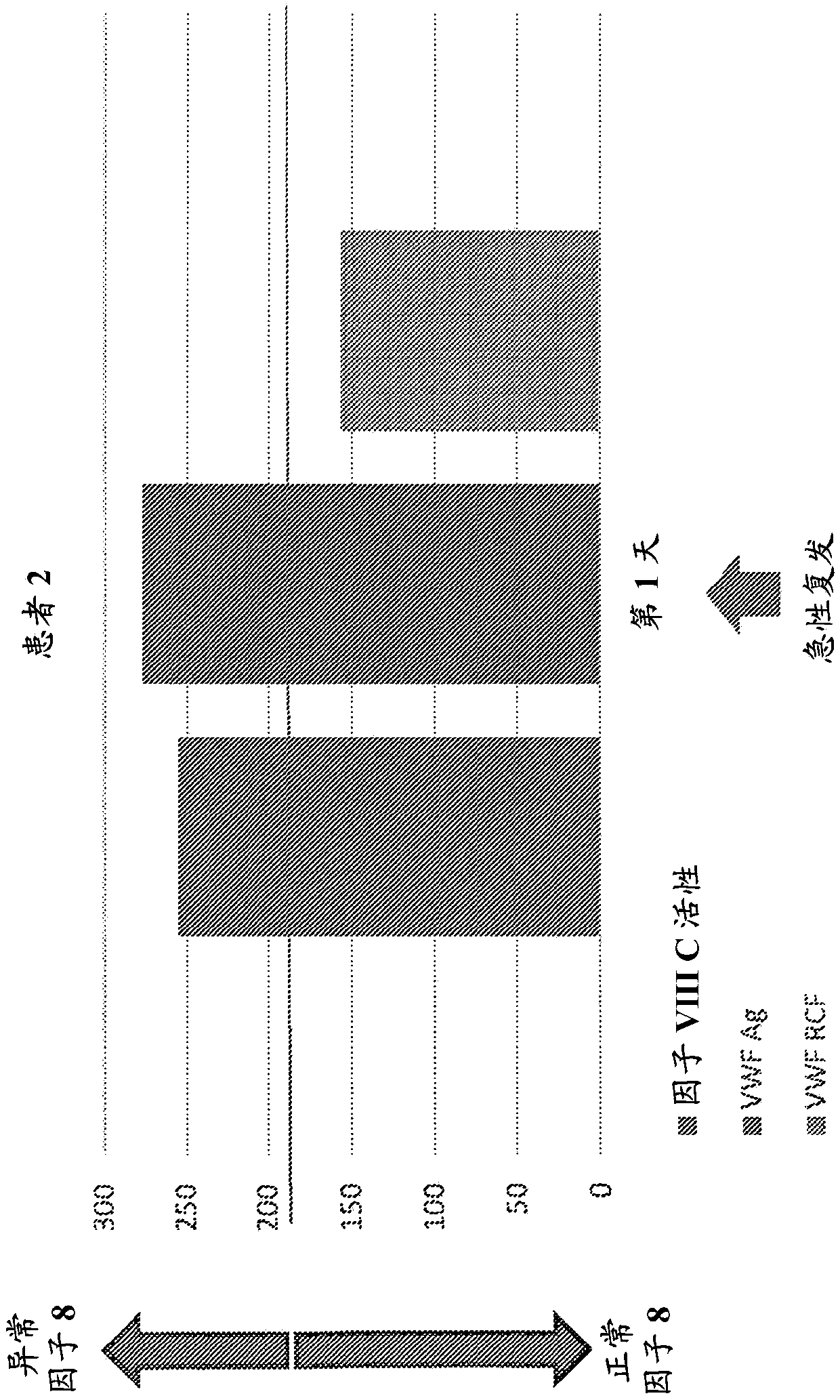
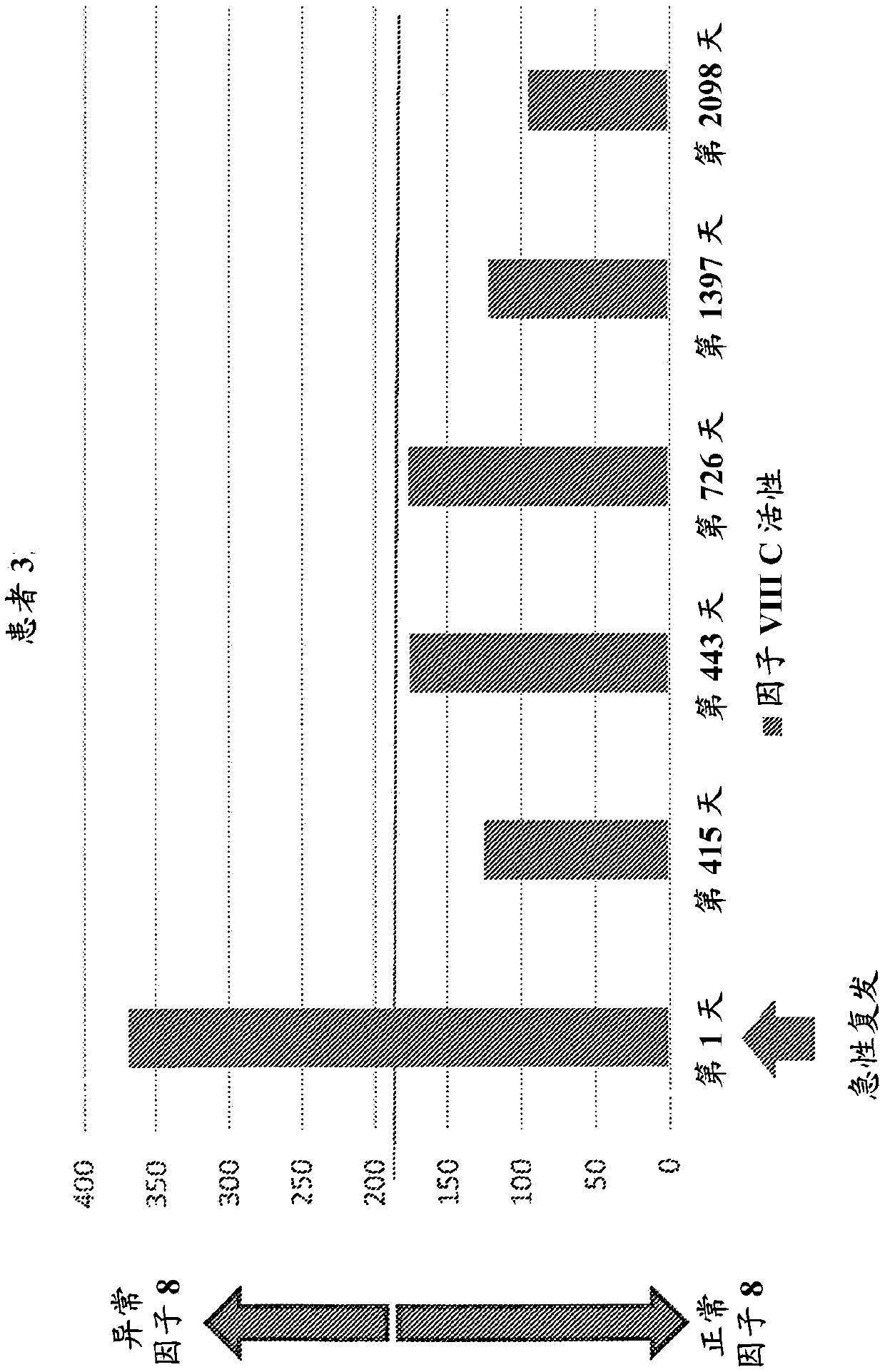
Diagnostic or predictor of relapsing remitting multiple sclerosis
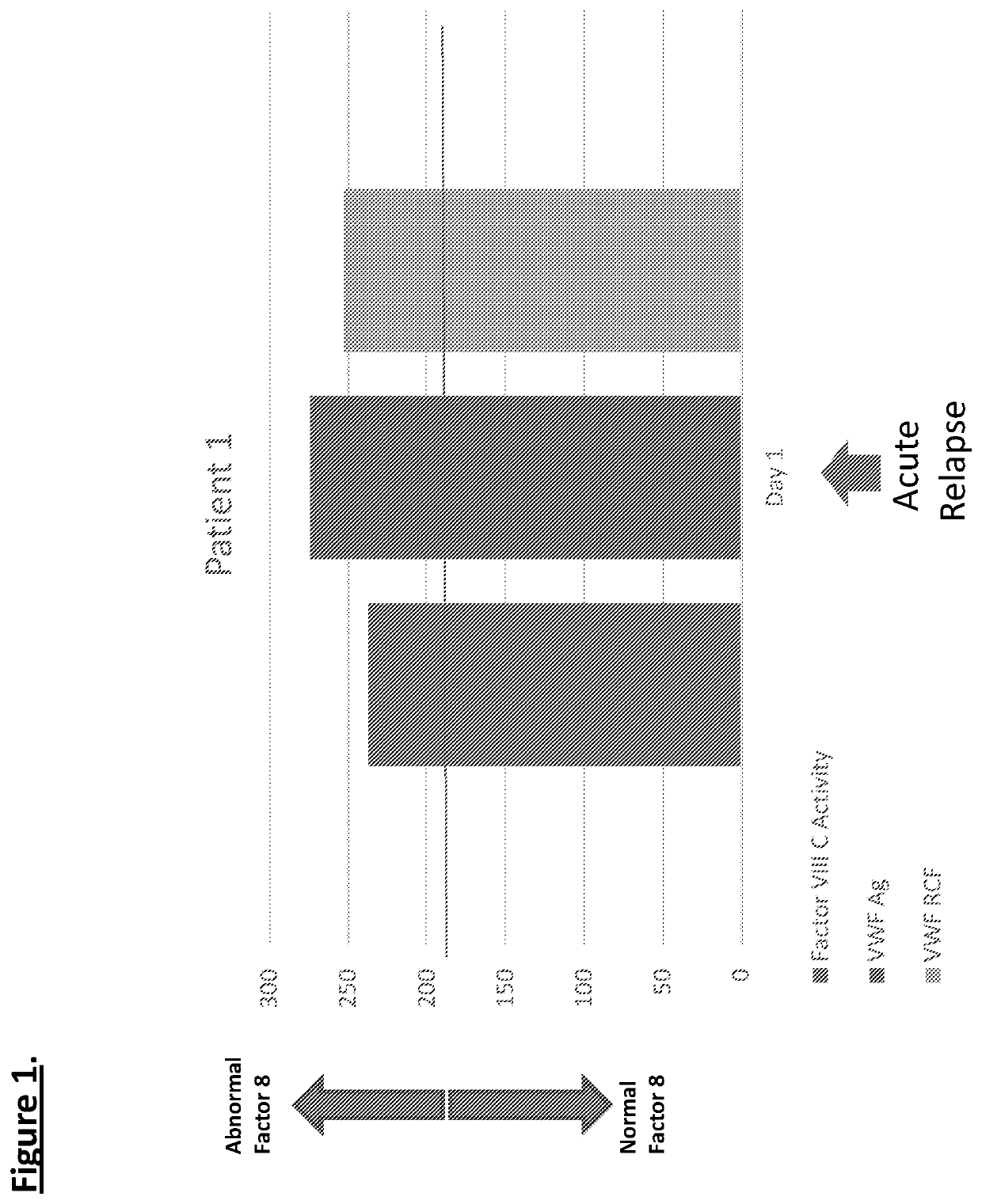
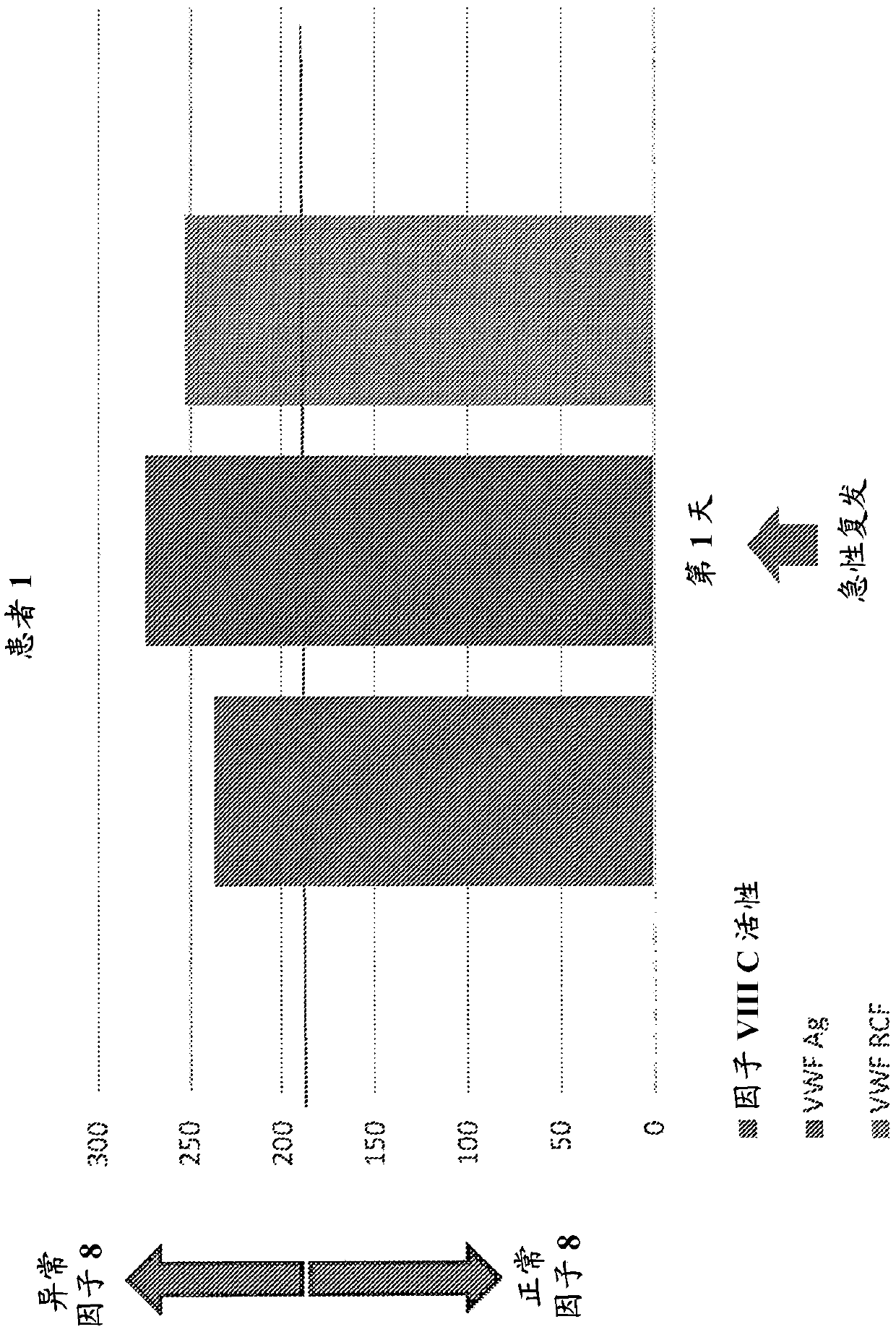
Provided herein is a method of detecting or predicting a relapse of multiple sclerosis in an individual afflicted with a form of multiple sclerosis, comprising: (a) providing a blood sample of the individual; (b) testing the blood sample to determine a protein activity or protein level, wherein the protein is Factor VIII, von Willebrand factor, or Protein C; and (c) detecting or predicting a relapse of multiple sclerosis in the individual if the protein activity or protein level is elevated compared to the protein activity or protein level in an individual not afflicted with the form of multiple sclerosis and patients' own baseline values. Also provided herein is a method of treating an individual afflicted with multiple sclerosis, who is experiencing a relapse or predicted to experience a relapse, comprising treating the individual by administering a dose of a steroid or anti-coagulation compound effective to alleviate the symptom of multiple sclerosis.
Owner:DIGNITY HEALTH
Assay and method for predicting therapeutic efficacy of immunoglobulin therapy in individual patients with relapsing remitting multiple sclerosis (rr-ms)
InactiveUS20150152498A1Prediction of responsivenessReliable predictionMicrobiological testing/measurementLibrary screeningDiseaseWhite blood cell
A method for generating a reference for determining the likelihood of response of a patient, suffering from a disease, towards immunoglobulin therapy comprising the steps ofa) providing samples of a sufficient number of individuals, in particular at least 10 individuals, the samples containing B- and T-lymphocytes, natural killer cells, invariant T-cells and monocytes of the individuals;b) determination of values of immune parameters which are either static, such as leukocyte subpopulations and cytokine-level in the plasma or functional, like gene expression and cytokine release after lipopolysaccharide (LPS) and / or IVIG stimulation ex vivo;c) the determined values derived from immune parameters of the samples of the individuals are ordered in quartiles and the values belonging to the 1. quartile, values distributed at the low end of the corresponding parameter, are set to “−1”, whereas values distributed in the 4. quartile are set to “+1” for an individual LDA-score calculation and values in between are set to “0”;d) genotyping of at least two of the polynucleotides selected from the group consisting of MTM1, EIF3E, COPS8, ADAMTSL1, CXXC4, RSPO2, OR9Q1, ADAMTS9, KLHDC8A and PRDM9, in the samples of the individuals and awarding the value of 0 for specific homozygous SNP combinations (SNP—Single Nucleotide Polymorphism), which indicates that the blood sample stems from a person which will respond to immunoglobulin (IG) treatment, while awarding the value of 1 for SNP combinations not meeting that criteria, which indicates that the blood sample stems from a person which will not respond to immunoglobulin treatment;e) combining the results of genotyping with results of immuno parameter determination in the calculation of an individual LDA-score (LDA—Linar Discriminant Analysis);f) combining a multiplicity of individual LDA-scores to create a reference Responder Score, which allows discrimination between responders and non-responders.The method can be employed for determining the likelihood of response of a patient, suffering from a disease, towards immunoglobulin therapy.
Owner:OCTAPHARMA +1
Laquinimod for the treatment of relapsing-remitting multiple sclerosis (RRMS) patients with a high disability status
InactiveUS20160074378A1Reducing ambulatory deteriorationHigh baseline disability scoreBiocideNervous disorderLaquinimodHuman patient
This invention provides a method for treating or for reducing ambulatory deterioration in a human patient diagnosed to be afflicted with relapsing-remitting multiple sclerosis (RRMS) and having a high baseline disability score according to the Kurtzke Expanded Disability Status Scale (EDSS), comprising periodically administering to only the patient diagnosed with RRMS and having a high baseline disability score an amount of laquinimod effective to treat the patient or to reduce ambulatory deterioration. This invention further provides pharmaceutical compositions and packages comprising an effective amount of laquinimod for treating a human patient diagnosed to be afflicted with RRMS and having a high baseline disability score according to the EDSS.
Owner:TEVA PHARMA IND LTD
Traditional Chinese medicine for multiple sclerosis
InactiveCN104873813AGood curative effectLow costHeavy metal active ingredientsNervous disorderLiver and kidneySecondary progressive
The invention relates to the field of medicine recipes, in particular to traditional Chinese medicine for multiple sclerosis. The traditional Chinese medicine is characterized by comprising the following active ingredients in parts by weight: 3-6 parts of caulis sinomenii, 4-6 parts of swertia mileensis, 3-7 parts of feather cockscomb seed, 4-6 parts of trametes versicolor, 4-6 parts of remote lemongrass herb, 2-4 parts of aplotaxis auriculata, 6-9 parts of glabrous sarcandra herb, 2-5 parts of radix saposhnikoviae, 5-7 parts of concha haliotidis, 2-5 parts of grassleaf sweelflag rhizome, 3-5 parts of chlorite schist, 3-5 parts of amethyst, 4-6 parts of radix ranunculi ternati, 1-2 parts of frankincense, 1-2 parts of scorpion, 2-5 parts of rhizoma cibotii, 2-4 parts of puncturevine, 2-4 parts of alisma, 2-5 parts of epimedium, 3-6 parts of radix semiaquilegiae, 2-4 parts of concretio silicea bambusae, 2-4 parts of cassia twig, 3-5 parts of fructus broussonetiae, 2-4 parts of longstamen onion bulb, 2-4 parts of stiff silkworm, and 2-4 parts of fried rhizoma arisaematis. The 26 active ingredients are soaked in water, and residues are removed after boiling to obtain a filter liquor. The traditional Chinese medicine for multiple sclerosis has the efficiencies of tonifying and replenishing liver and kidney, dredging stagnating, reducing phlegm, nourishing yin and blood, replenishing qi, strengthening the spleen, reducing fever, drying moistening, detoxicating, eliminating evil, draining dampness, dissolving turbidity, blending yin and yang, reinforcing the vital essence and strengthening the primordial qi, and is effective to relapsing-remitting, secondary progressive, primary progressive, and progressive-relapsing courses of disease.
Owner:宋春雨
Sphingosine-1-phosphate receptor agonist, process for its preparation and pharmaceutical composition comprising same as active agent
ActiveCN105051037BPrevent immunomodulatory disordersOrganic active ingredientsNervous disorderAutoimmune conditionActive agent
The present invention relates to novel compounds of formula 1 as sphingosine-1-phosphate receptor agonists which can be effectively used in the treatment of autoimmune diseases, processes for the preparation of said compounds and comprising said compounds as active ingredients pharmaceutical composition. The compounds of the invention are effective against a wide range of autoimmune and chronic inflammatory diseases, including relapsing remitting multiple sclerosis, and can be used to treat or prevent immunomodulatory disorders.
Owner:LG CHEM LTD
Marker of Diagnosis and Prognosis in Multiple Sclerosis
InactiveUS20130157270A1Increase the number ofMicrobiological testing/measurementMaterial analysisRelapsing remittingGene
The present invention provides a method for determining whether an individual with relapsing-remitting multiple sclerosis will suffer a relapse. In the method, measuring the level of Response Gene to Complement (RGC)-32 is measured in the individual, where a significantly lower level of RGC-32 therein indicates that the individual will have or is having a relapse of multiple sclerosis.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Polymeric compositions of monomethyl fumarate and their use in treating relapsing remitting multiple sclerosis and psoriasis
ActiveUS20200237704A1Reduce gastrointestinal side effectsIncrease concentrationOrganic chemistryPharmaceutical delivery mechanismPoly-L-lactideLactide
The present invention comprises compounds of formula (I): where R1 may be PEG or other polymeric moieties. For example, R1 may be repeating PEG units —(CH2—CH2—O)n—, wherein n=1-455. R1 may also be other polymeric moieties of varying sizes and structures, for example, R1 may be poly(glycolide), poly(lactic acid), poly(lactide), poly(caprolactone), poly(lactide-co-caprolactone), poly(lactide-co-glycolide), or poly(lactic acid)-butanol. One or more embodiments of the invention may also relate to injectable pharmaceutical compositions comprising polymer conjugated monomethyl fumarate, and methods for treating relapsing-remitting multiple sclerosis and psoriasis.
Owner:DYNAMIC BIOLOGICS INC
Diagnostic or predictor of relapsing remitting multiple sclerosis
Provided herein is a method of detecting or predicting a relapse of multiple sclerosis in an individual afflicted with a form of multiple sclerosis, comprising: (a) providing a blood sample of the individual; (b) testing the blood sample to determine a protein activity or protein level, wherein the protein is Factor VIII, von Willebrand factor, or Protein C; and (c) detecting or predicting a relapse of multiple sclerosis in the individual if the protein activity or protein level is elevated compared to the protein activity or protein level in an individual not afflicted with the form of multiple sclerosis and patients' own baseline values. Also provided herein is a method of treating an individual afflicted with multiple sclerosis, who is experiencing a relapse or predicted to experience arelapse, comprising treating the individual by administering a dose of a steroid or anti-coagulation compound effective to alleviate the symptom of multiple sclerosis.
Owner:DIGNITY HEALTH
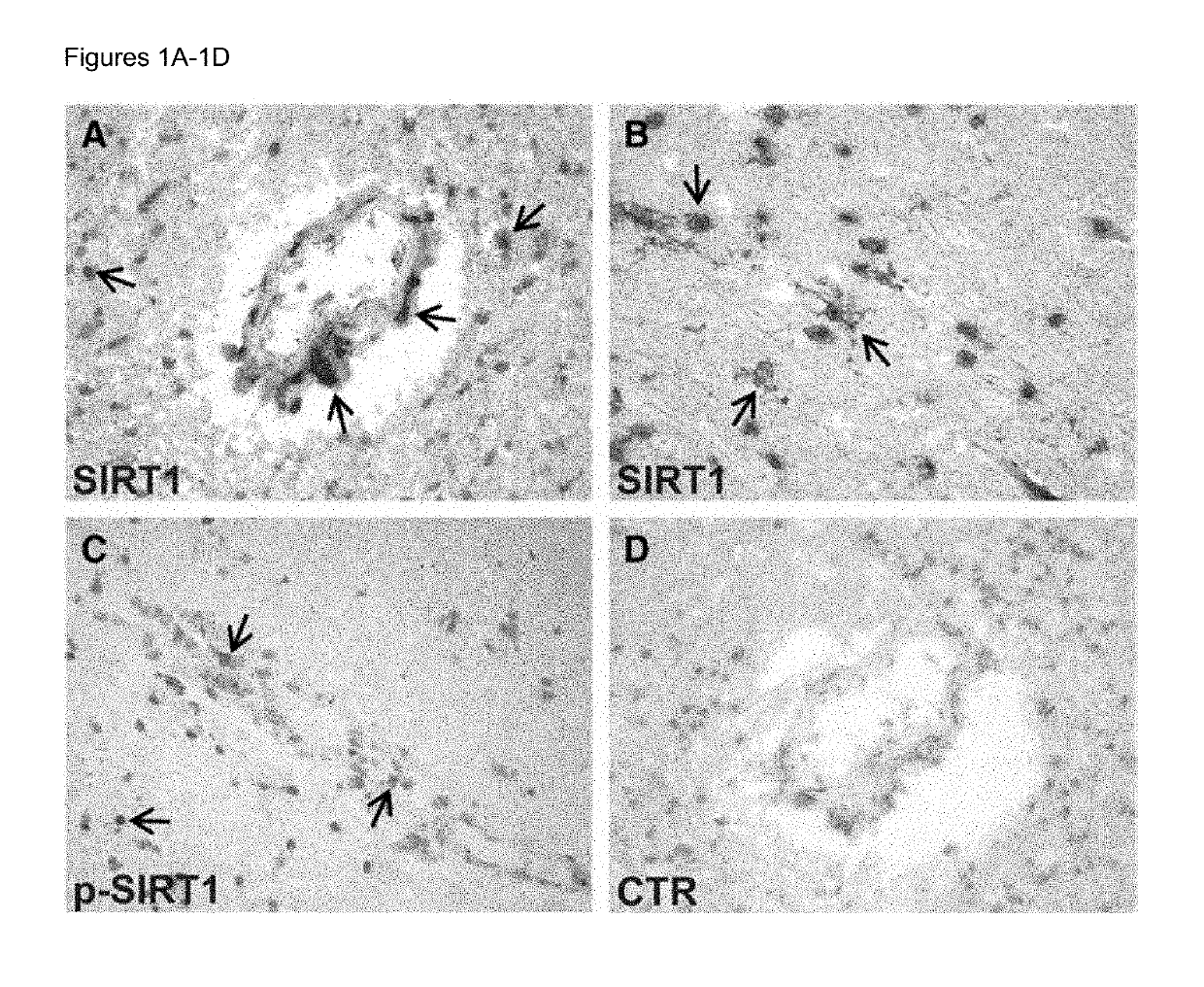
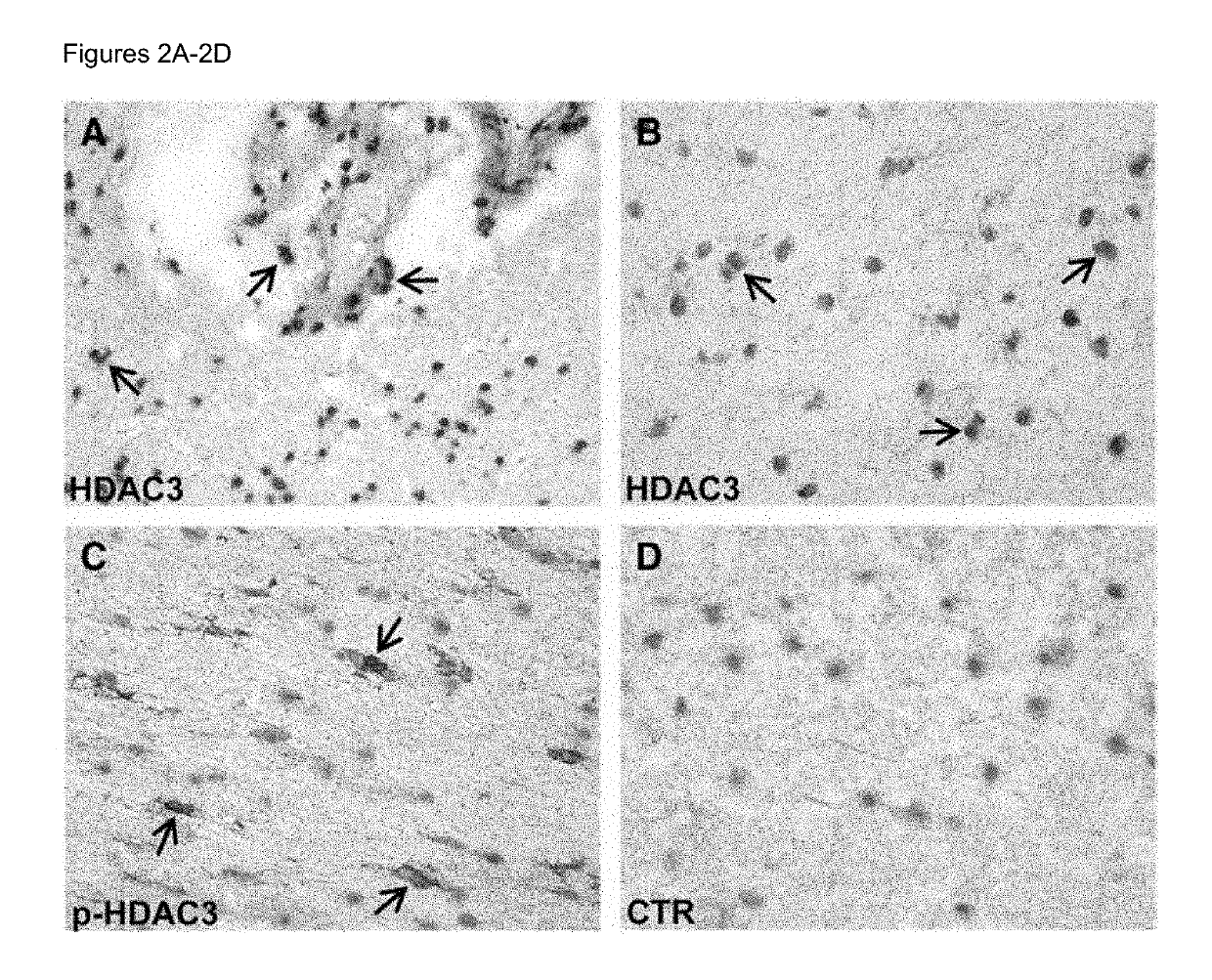
Biomarkers for predicting relapse in multiple sclerosis
ActiveUS10280465B2Microbiological testing/measurementDisease diagnosisBiomarker (petroleum)Relapsing remitting
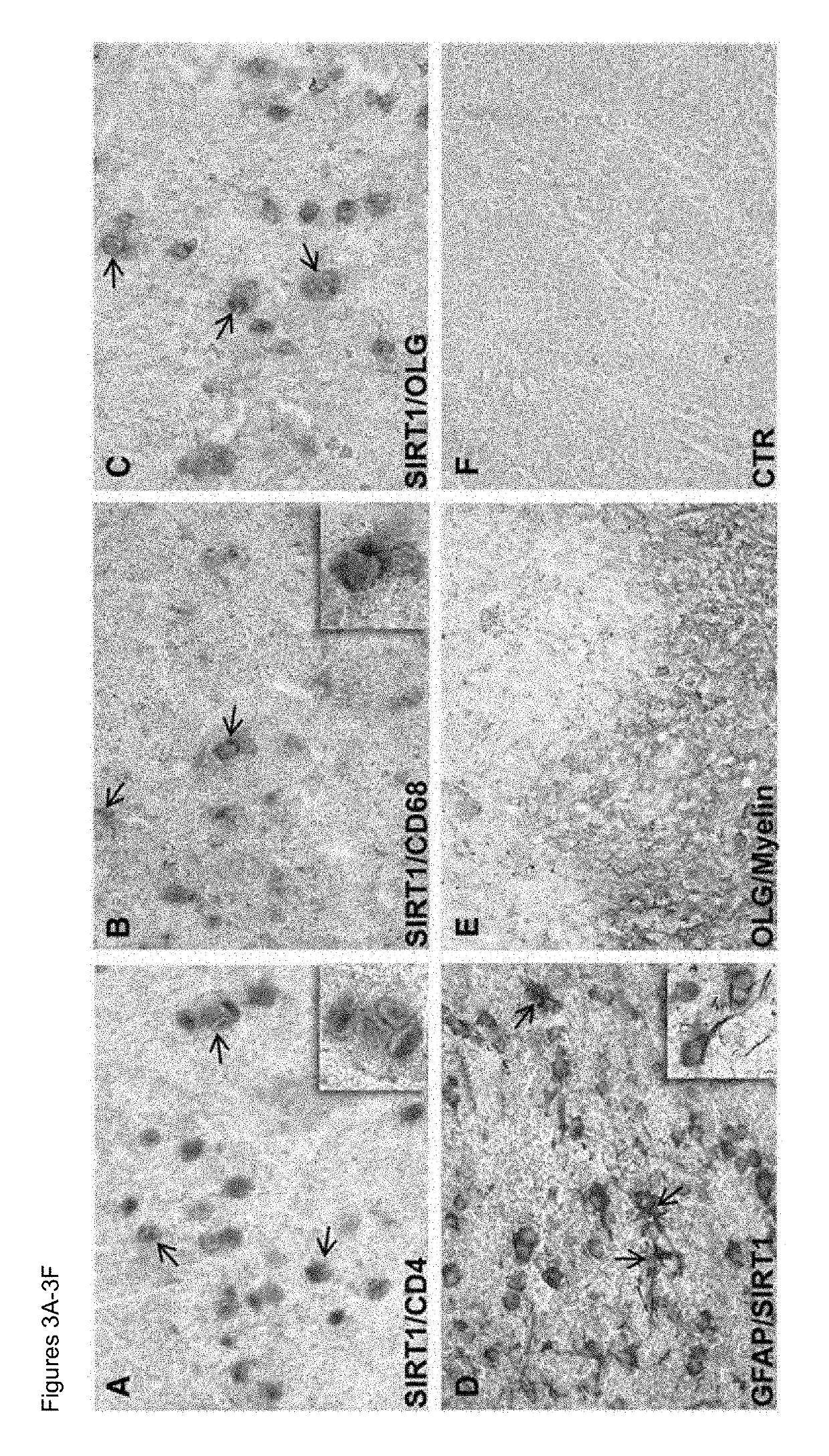
Methods of determining relapse in subjects having relapsing-remitting multiple sclerosis (RRMS) and predicting their response to glatiramer acetate (GA) using the biomarkers SIRT1, RGC-32, FasL and IL-21 are presented.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Application of combination of cladribine and glatirameracetate in treating multiple sclerosis
The invention relates to a method of treating relapsing-remitting multiple sclerosis by combining multi-dose cladribine and glatirameracetate for a needed patient.
Owner:TIANJIN HANKANG PHARMA BIOTECH
N-acetyl glucosamine as a biomarker of MS disease course
The invention provides the art with a powerful diagnostic method of distinguishing relapse-remitting MS subjects from progressive MS subjects, based on the measurement of serum concentrations of N-acetylglucosamine (GlcNAc,), for the first time enabling rapid diagnosis of the progressive form of MS. GlcNAc serum concentration can also be used to assess neurodegenerative status and MS progression in subjects suffering from MS or other neurological conditions. The methods of the invention also allow for the identification of new therapeutics for MS and other neurological conditions and also enables the personalized efficacy assessment of a potential therapy for an MS subject.
Owner:CHARITE UNIVS MEDIZIN BERLIN +2
Diagnosis and Prognosis of Multiple Sclerosis
ActiveUS20160208332A1Accurate detectionAccurately detecting responseMicrobiological testing/measurementLibrary screeningCut off valueCvd risk
The present invention provides a method for determining whether an individual with relapsing-remitting multiple sclerosis will suffer a relapse or respond to treatment for MS. A ratio of mRNA levels of Response Gene to Complement-32, FasL or IL-21 to L13 determined for an individual provides a normalized level which is compared to a cut-off value. A normalized level of Response Gene to Complement-32 greater than 2.52, a normalized level of FasL greater than 85.4 and a normalized level of IL-21 less than 11.9, respectively, indicates the individual will have or is having a relapse of multiple sclerosis. Also provided are methods for determining whether an individual will respond positively or is responding positively to glatiramer treatment and whether the individual is in a period of stable disease or is not at risk for relapse of multiple sclerosis by comparing normalized levels with the respective cut-off levels.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com