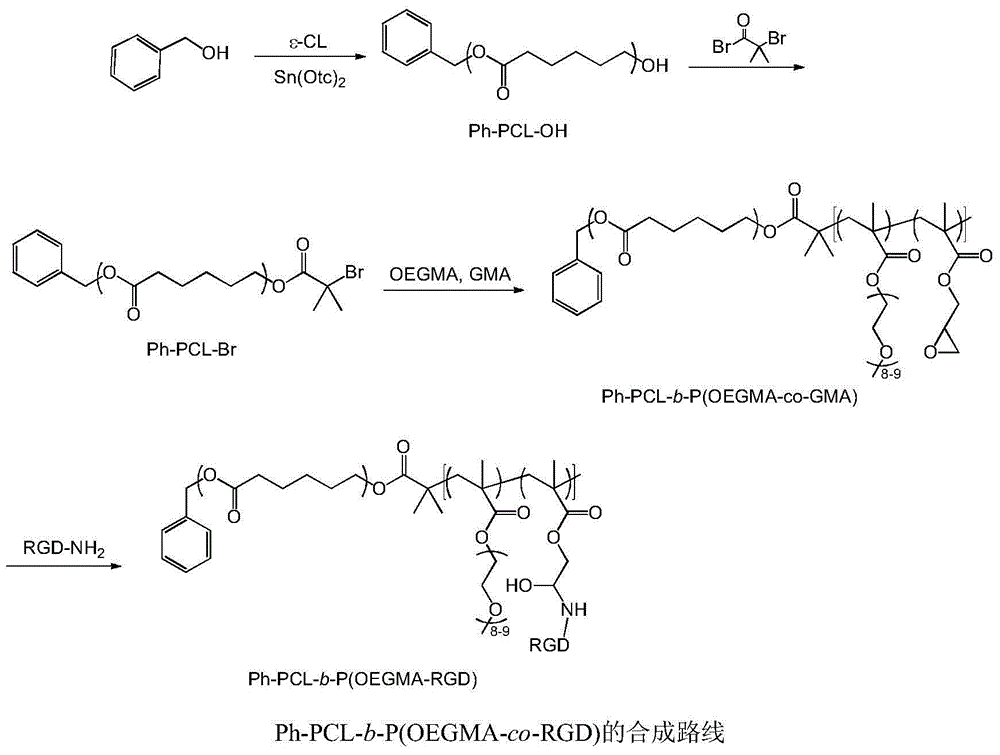
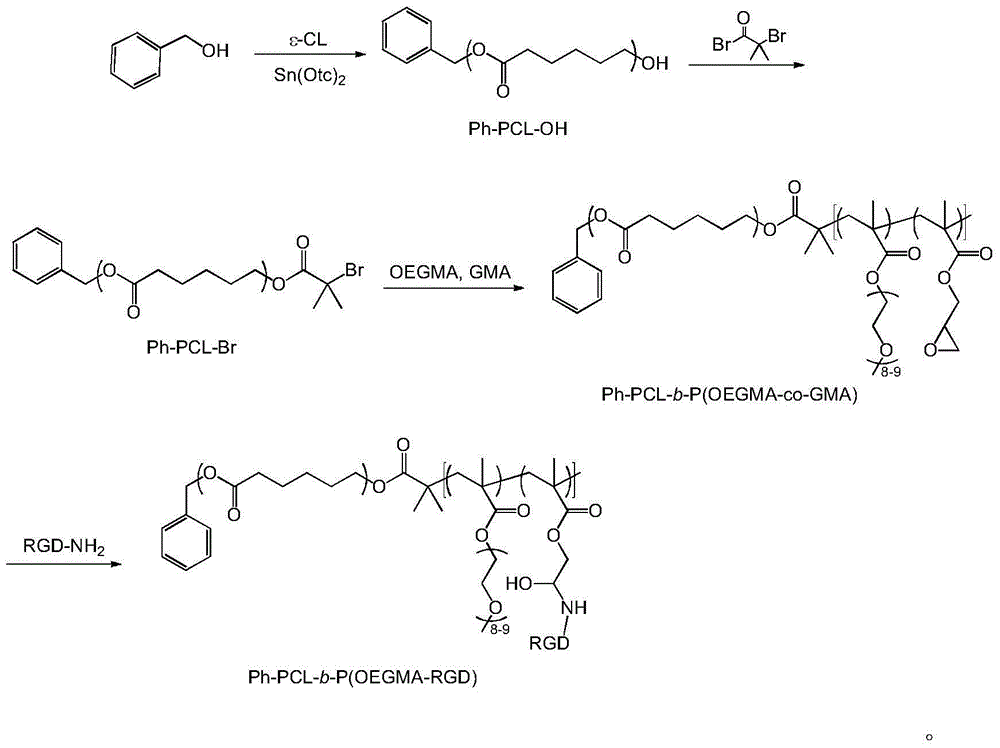
Bufalin-loaded polypeptide-modified poly(oligo(ethylene glycol)methacrylate)-polycaprolactone (Ph PCL b P(OEGMA co RGD) bufalin) nanometer preparation
A technology of polymethacrylic acid and polyethylene glycol ester, which is applied in the directions of inactive medical preparations, antitumor drugs, and emulsion delivery, etc., can solve problems such as unpublished, inconvenient nanomaterials, and inability to use molecular weight calculations. , to achieve good targeting ability, improve water solubility, and improve the effect of tumor targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of valine-arginine-glycine-aspartic acid-glutamic acid cyclic peptide (cRGD) modified poly(ethylene glycol methacrylate)-polycaprolactone nanoparticles (Ph-PCL- b -P(OEGMA- co -RGD)-bufalin)
[0040] (1) Preparation of Ph-PCL-OH: Vacuum heating and drying of heat-resistant glass tube, add 10g caprolactone and 95mg benzyl alcohol, then add 20 mu L stannous octoate, heat to dissolve and vacuumize, cool and solidify and vacuumize for 2 hours, then seal the tube, 130 o C was reacted for 5h, and purified by precipitation in ice methanol;
[0041] (2) Preparation of Ph-PCL-Br: Dissolve 4.0g of Ph-PCL-OH and 1mL of triethylamine in an organic solvent, transfer to an ice-water bath, then add 1mL of 2-bromoisobutyryl bromide dropwise into the solution, and react After 3 days, the reaction product can be purified: filter, wash with alkali and water or dialyze, concentrate, add ice methanol to precipitate the product, filter, and vacuum dry;
[0042] (3) Preparati...
Embodiment 2
[0046] Prepare nanoparticle solution by double emulsion method, take 4mgPh-PCL- b -P(OEGMA- co -RGD) was dissolved in 200 μL of dichloromethane or a mixed solvent of dichloromethane and acetone, adding 0.2 mg of bufalin drug solution, ultrasonic emulsification (400W, 10sx4), and then adding 2.2 mL of 1% Pluronic F68 aqueous dispersion medium , ultrasonic emulsification (400W, 10sx4) again, and then stirred at room temperature for 0.5-5h to remove the organic phase to obtain a nanoparticle solution.
Embodiment 3
[0048] The nanoparticle solution was prepared by film emulsification method, and 4mgPh-PCL- b -P(OEGMA- co -RGD) and 0.2 mg of bufalin drug were dissolved in 400 μL of acetone solvent, rotatively evaporated to form a film, then 4 mL of aqueous solution was added, and stirred at room temperature for 0.5-6 h to obtain a nanoparticle solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com