Targeted antibacterial peptide against Gram-negative bacteria and preparation method and application
A gram-negative bacterium and a technology for making it, applied in the biological field, can solve the problems that gram-negative bacteria have no effect and no medicine is available, and achieve the effects of reduced screening costs, high application potential, and low hemolytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Use the R language to statistically analyze the amino acid sequence characteristics of the natural antibacterial peptide library, and perform statistical analysis on the peptide chain parameters that affect the antibacterial activity of the peptide chains that only target Gram-negative bacteria, and screen to obtain the best amino acid composition, namely K(lys), G(Gly), L(Leu); number of positive charges: 4, hydrophobicity: 30%-50%. On this basis, combined with the study of structure-function relationship, tryptophan was inserted into the center of the screening sequence to design a centrosymmetric short peptide sequence; to obtain a short-chain narrow-spectrum antimicrobial peptide sequence with low toxicity, high efficiency, and less likely to cause immune regulation disorders.
[0019] Amino acid sequence of table 1 antimicrobial peptide
[0020]
Embodiment 2
[0022] Synthesis of pheromone-labeled antimicrobial peptides by solid-phase chemical synthesis
[0023] 1. The preparation of antimicrobial peptides is carried out one by one from the C-terminal to the N-terminal, and is completed by a peptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminal of each antimicrobial peptide) is inserted into Wang resin, and then the Fmoc group is removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fmoxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antimicrobial peptide); according to this procedure, it is synthesized from the C-terminus to the N-terminus until the synthesis is completed, and the side of the Fmoc group is removed chain protection resin;
[0024] 2. Add a cleavage reagent to the peptide resin obtained above, react for 2 hours at 20°C in the dark, filter; wash the precipitate with TFA (trifluoroacetic acid), mix the washing liquid with the above filtrate, concentrate with a rotary evap...
Embodiment 3
[0028] Determination of Antimicrobial Peptide Activity
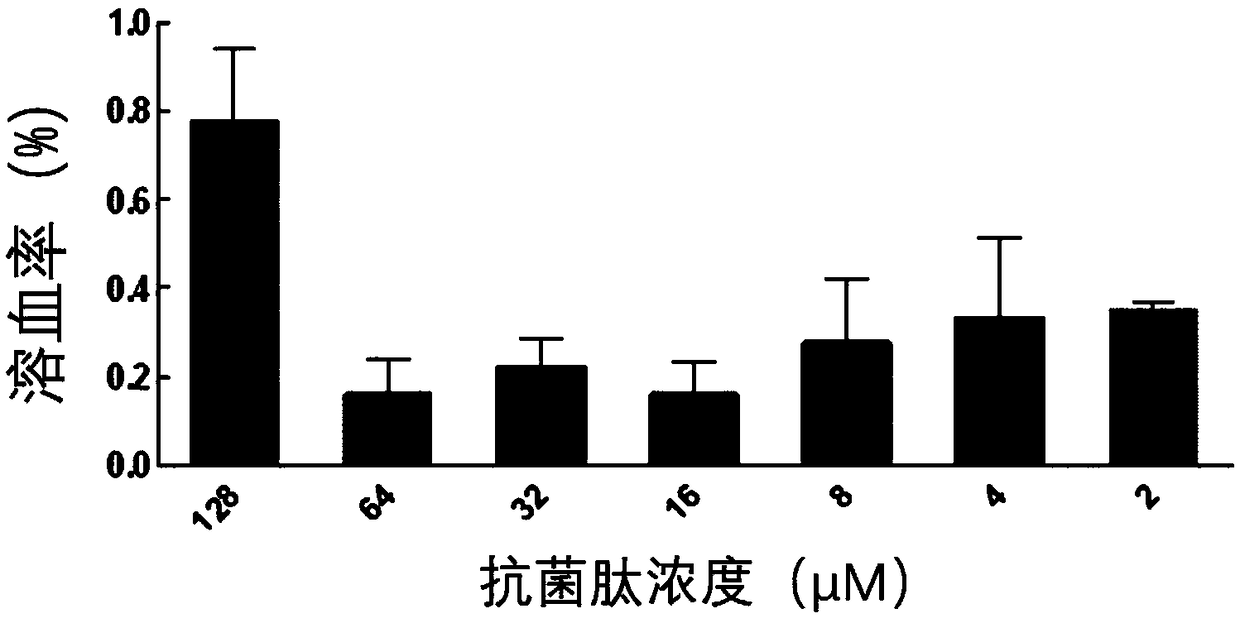
[0029] 1. Determination of antibacterial activity: the antimicrobial peptides are configured into a certain storage solution for use. The minimum inhibitory concentrations of several antimicrobial peptides were determined by the broth microdilution method. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 individual / mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Incubate at a constant temperature of 37°C for 20 hours, and the minimum inhibitory concentration is the one where no turbidity is seen at the bottom of the well with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrophobicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com