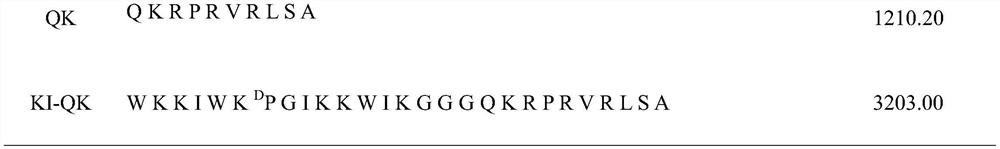Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about How to "Simple experimental technique" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Simple preparation method of hollow-spherical and flower-shaped indium oxide with secondary structure and application
InactiveCN102134092AWide variety of sourcesSimple experimental techniqueChemiluminescene/bioluminescenceGallium/indium/thallium compoundsMicrosphereActive agent
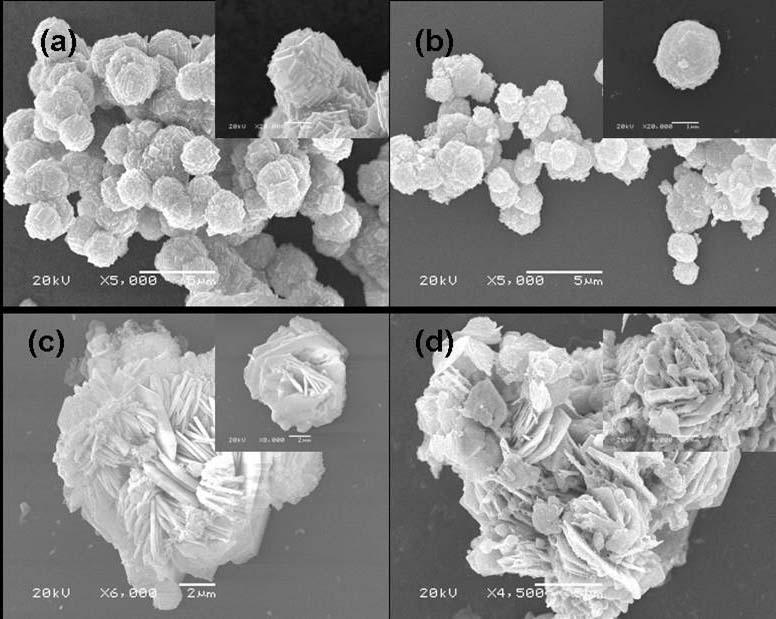
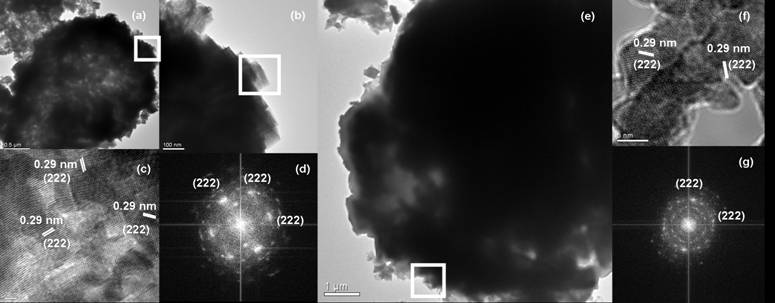
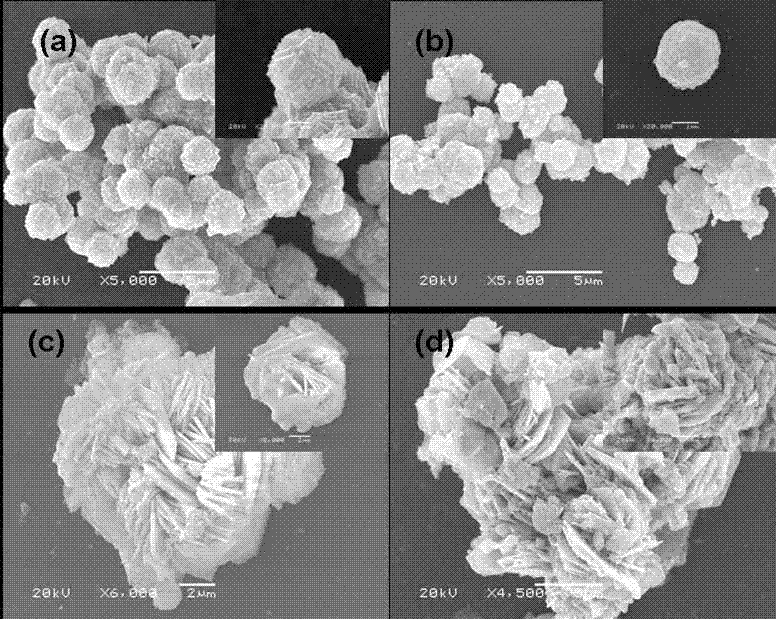
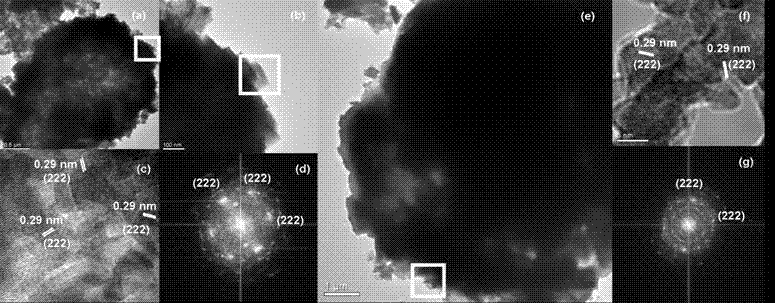
The invention provides a preparation method of indium oxide with new appearance, belonging to the technical field of synthesis and nano material of the indium oxide. The preparation method comprises the following steps: taking inorganic salt solution of the indium as an indium source, adopting N, N-dimethyl formamide (DMF) as an alkali source and anion active agents such as sodium dodecyl sulfate(SDS) and the like as an additive, using hydrothermal synthesis to prepare the novel hollow-microspherical and flower-shaped indium oxide with the secondary structure under the condition without adding any hard template, and controlling the appearance of the secondary structure by chemical means. In the preparation method provided by the invention, the technical process is simple, the promotion is easy, the selected reagent is low in price and environment-friendly, and the need on low-cost, large-scale and green production is met.
Owner:SICHUAN UNIV
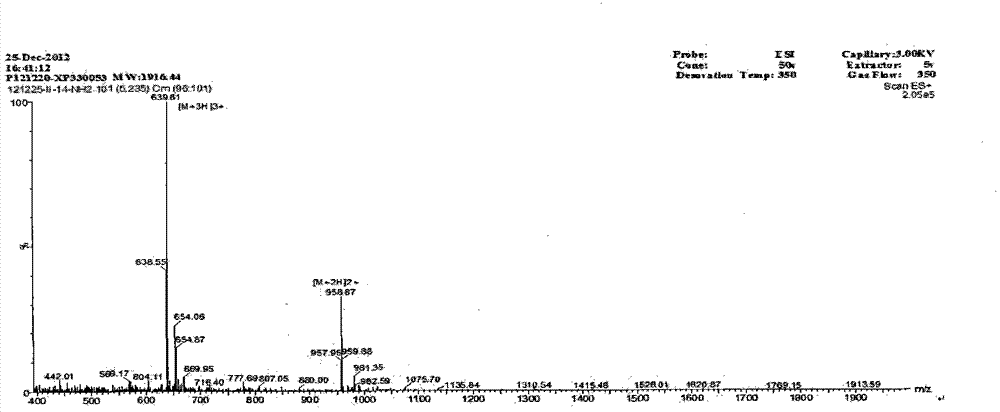
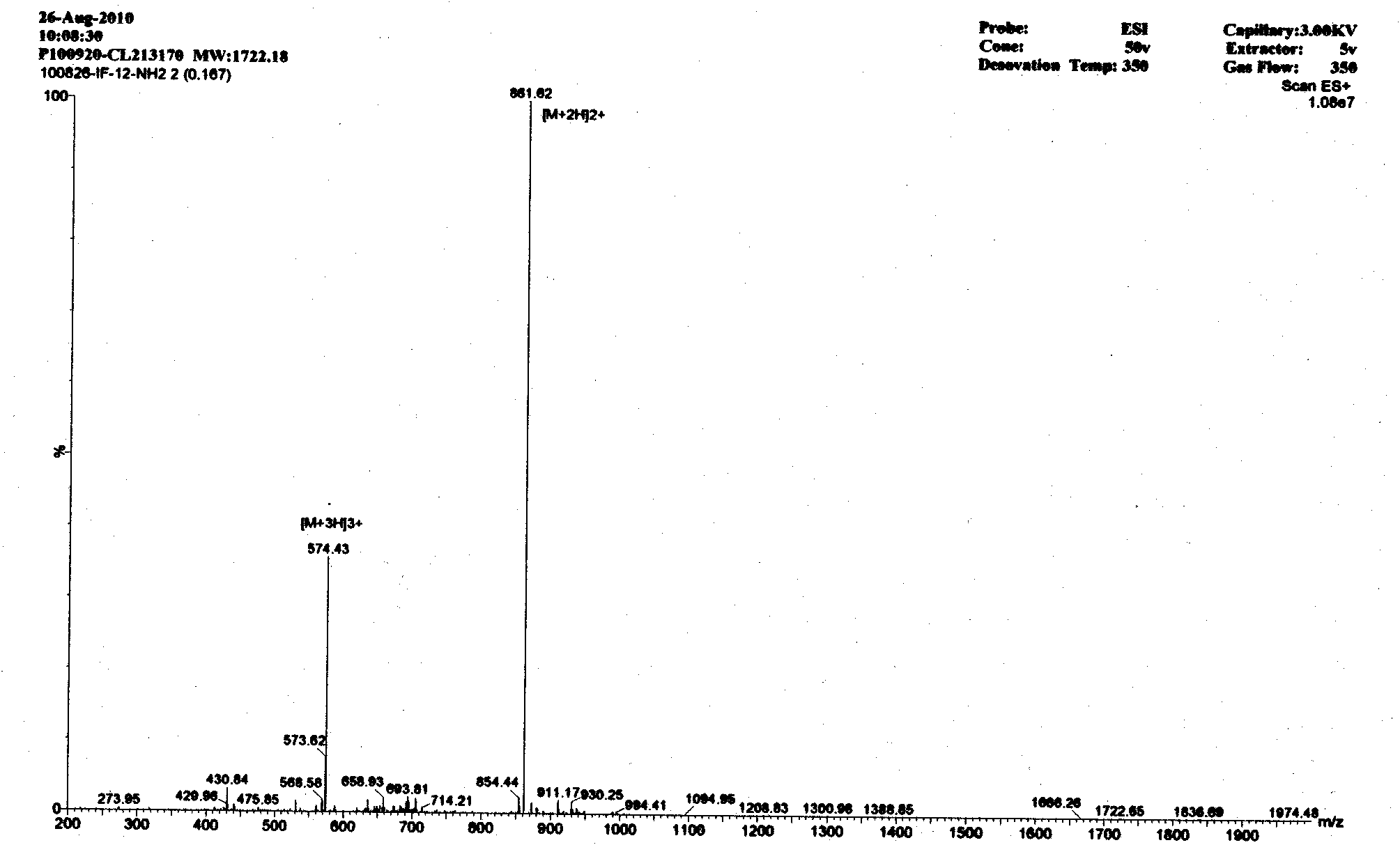
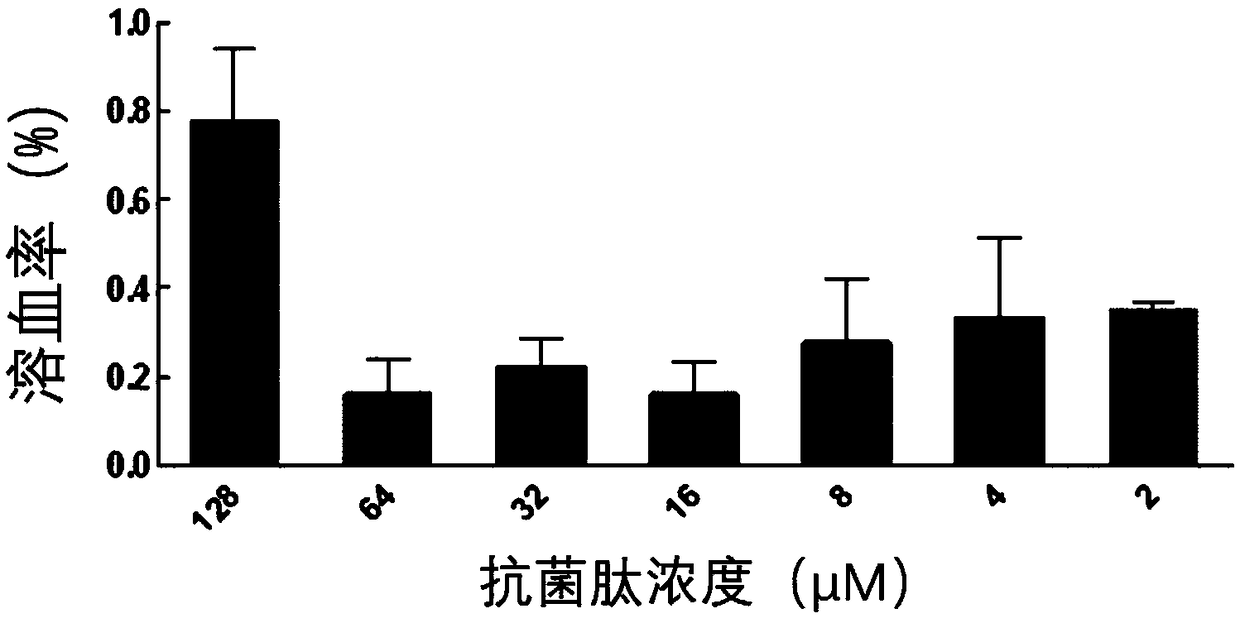
Derived peptide IR2 of pig-derived antibacterial peptide as well as preparation method and application thereof
ActiveCN103923189ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsArginineAntibacterial activity
The invention provides a derived peptide IR2 of a pig-derived antibacterial peptide as well as a preparation method and application thereof. The sequence of the derived peptide IR2 of the pig-derived antibacterial peptide is as shown in SEQ ID No.1. Antibacterial peptides IR1 and IR2 are obtained by using a fixed point amino acid fragment interception and binary amino acid sequence superimposing method for intercepting six amino acid fragments of a pig-derived PG-1 corner part and symmetrically and circularly arranging charged amino acid arginine and hydrophobic amino acid isoleucine serving as repeated binary sequence units at the two sides of the corner. The antibacterial activity of the antibacterial peptide IR2 is higher than that of the antibacterial peptide IR1. The therapeutic index of the antibacterial peptide IR2 reaches up to 43.2 and is 216 times of that of the pig-derived antibacterial peptide RG-1. By virtue of the method, the hemolytic activity of the antibacterial peptide is greatly reduced under the condition of increasing the antibacterial activity of the antibacterial peptide, the selectivity of the antibacterial peptide between bacterial cells and mammalian cells is increased, and the development potential of the antibacterial peptide serving as antibiotic substitutes is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
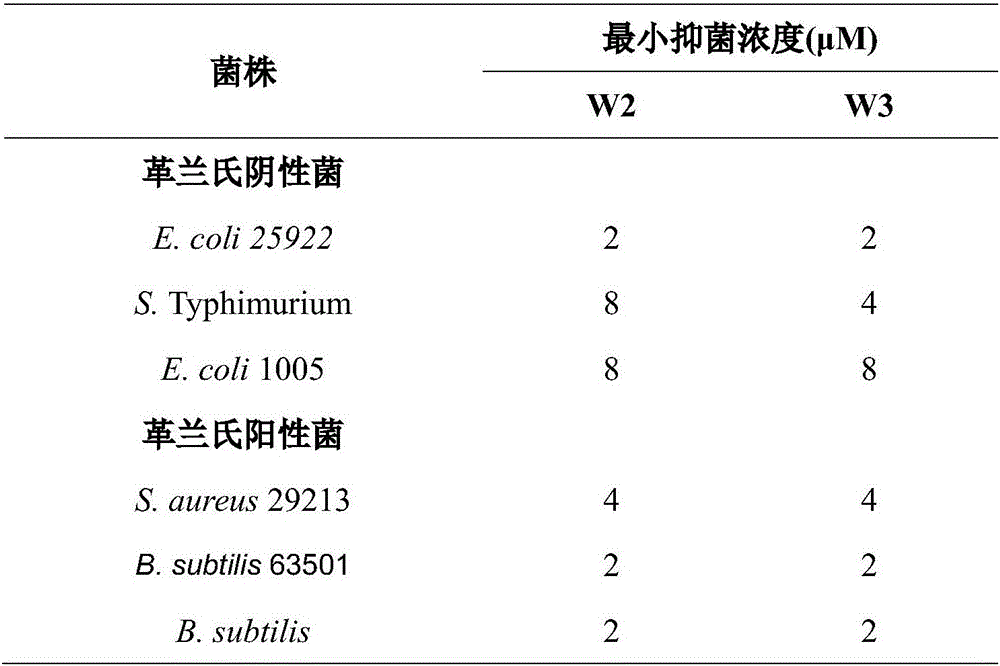
Multi-stranded beta-hairpin short-peptide with tolerant protease and preparation method and application
InactiveCN106749532ASimple experimental techniqueStable structureAntibacterial agentsPeptide/protein ingredientsBeta hairpinCrystallography
The invention provides a multi-stranded beta-hairpin short-peptide with tolerant protease and a preparation method and an application. A sequence is as shown in a SEQ ID No.1. The short-peptide provided by the invention fully exerts the structural advantages of a beta-hairpin structure to obtain a multi-stranded beta-hairpin structural antibacterial peptide. The stability is further improved while the activity is ensured by using a common amino, and composition of a corner amino acid is changed, so that a series of multi-stranded beta-hairpin antibacterial peptides of brand new structures: (WRXxRW)n-NH2, wherein n is 1, 2, 3 and 4; PG is selected as a corner to describe; when n is equal to 2, the antibacterial peptide is named W2; and when n is equal to 3, the antibacterial peptide is named W3. The peptide verifies relatively high cell selectivity and salt ion tolerance, and moreover, based on the activity of the antibacterial peptide with structural stability, the obtained antibacterial peptide is also relatively short, and relatively strong tolerance of in vivo protease is also represented.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Tryptophan tension chain-beta-hairpin antibacterial peptide and preparation method and application thereof
InactiveCN106749531ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsCell selectivityCytotoxicity
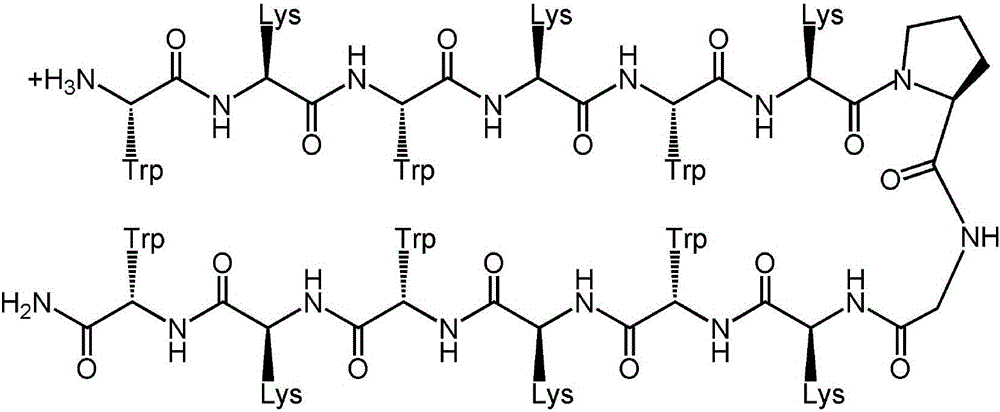
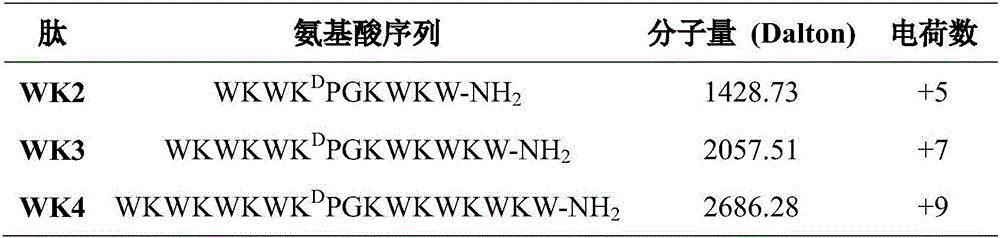
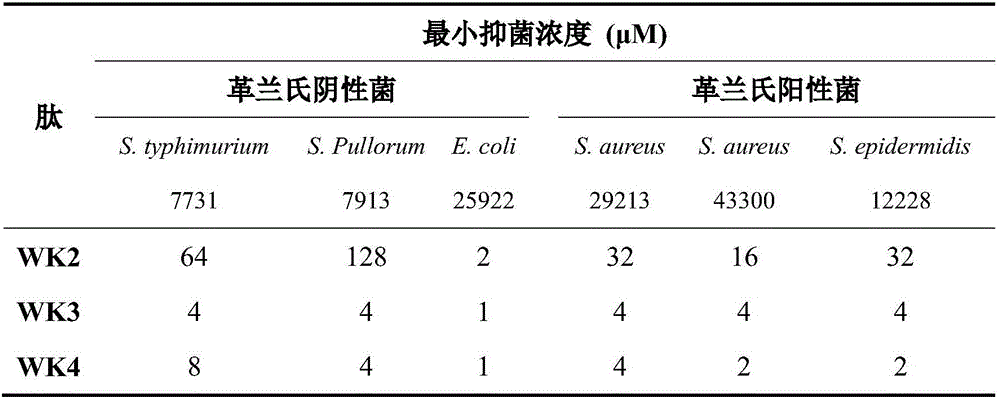
The invention provides a tryptophan tension chain-beta-hairpin antibacterial peptide and a preparation method and application thereof. A sequence of the antibacterial peptide is as shown in a sequence table SEQ ID No.1. The preparation method comprises the following steps: by taking a tryptophan tension chain structure as a template, combining amino acid composition and structural characteristics of the antibacterial peptide; and by using interaction of cross-chain W-W as a structural stable factor, symmetrically and circularly arranging an amino acid lysine with charges and a hydrophobic amino acid tryptophan as repeated binary sequence units on two arms of the beta-hairpin to obtain the antibacterial peptide WK3. The antibacterial peptide has relatively high bacteriostatic activity and cell selectivity, and the treatment index reaches up to 161.27. The antibacterial peptide designed by the method needs not to bind a disulfide bond but has extremely high structural stability, so that the selectivity of the antibacterial peptide between bacterial cells and mammalian cells is improved, and the antibacterial peptide has an application potential as an antibiotic substituent. The antibacterial peptide is relatively high in activity and relatively low in cytotoxicity.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Improved mitochondrial genome complete sequence determination method
InactiveCN101875966ALow costReduce technical difficultyMicrobiological testing/measurementPcr methodComplete sequence
The invention discloses an improved mitochondrial genome complete sequence determination method based on the conventional PCR technology, which aims at providing a low-cost, fast and new mitochondrial genome complete sequence determination method having a simple experimental technology. The method is characterized by the screening of templates and the design of PCR primers, i.e determining the minimal homology of mitochondrion complete sequences of closely-related species which can be used as the templates; carrying out primer design in two batches, and introducing a nested-PCR method to the sequence amplification process; and presenting general rules for the design of the PCR primers. The method is mainly used for mitochondrial genome complete sequence determination, and serves basic scientific researches, such as genetics, molecular biology and the like.
Owner:HAINAN UNIVERSITY
A kind of antibacterial peptide and preparation method thereof
InactiveCN102276691ASimple experimental techniqueImprove therapeutic indexAntibacterial agentsPeptide preparation methodsCell selectivityArginine
The invention relates to antibacterial peptides and a preparation method thereof. An amino acid sequence of the antibacterial peptide is IleTrpArgIlePheArgArgIlePhe. The method comprises the following steps of: 1) designing a group of antibacterial peptides consisting of isoleucine, arginine and phenylalanine which serve as basic units by utilizing the bacteriostatic mechanism of the antibacterial peptides and the characteristic of amino acid composition; 2) synthesizing a polypeptide crude product by using a polypeptide synthesizer in a solid-phase synthetic process; 3) purifying the synthesized polypeptide by reversed phase high performance liquid chromatography, and identifying by electrospray mass spectrometry; and 4) performing an in-vitro activity test, wherein a result of the activity test indicates that antibacterial peptides B containing 3 basic units have high bacteriostatic activity and weak hemolytic activity, a treatment index is the highest, and cell selectivity is optimum, so the antibacterial peptides have high application value.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Targeted antibacterial peptide against Gram-negative bacteria and preparation method and application
ActiveCN109232717ASimple experimental techniqueHigh application potentialAntibacterial agentsPeptide/protein ingredientsStatistical analysisCytotoxicity
The invention provides a targeted antibacterial peptide against Gram-negative bacteria and a preparation method and an application. A sequence of the targeted antibacterial peptide is as shown in a sequence table SEQ ID No. 1, and in the preparation method, an R language is utilized for statistical analysis of amino acid sequence characteristics of a natural antimicrobial peptide library, variouspeptide chain parameters affecting antibacterial activity of a peptide chain having the antibacterial activity only against Gram-negative bacteria are statistically analyzed, and the optimal amino acid composition is obtained by screening, namely K, G, L, with the number of positive charges being 4, and the hydrophobicity being 30%-50%. Tryptophan is inserted into the center of a screening sequence to obtain a centrally symmetric short peptide sequence through design, so as to obtain a short-chain narrow-spectrum antibacterial peptide sequence which is low in toxicity and highly effective, andnot easy to cause disorder of immune regulation in a body. The targeted antibacterial peptide is beneficial to maintaining micro-ecological balance while killing harmful bacteria. The antibacterial peptide has relatively high activity, high stability, relatively low cytotoxicity, and substitute antibiotics, thereby having an application potential for becoming a safer and environmentally-friendlynovel antibacterial product.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
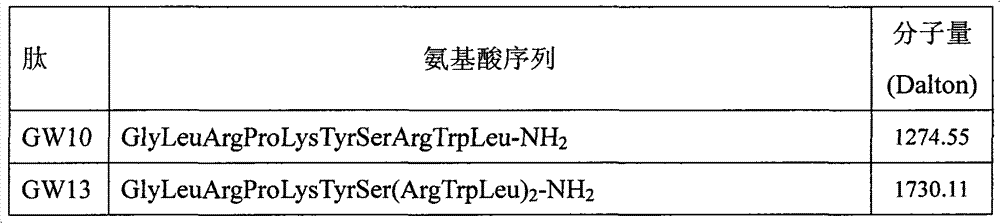
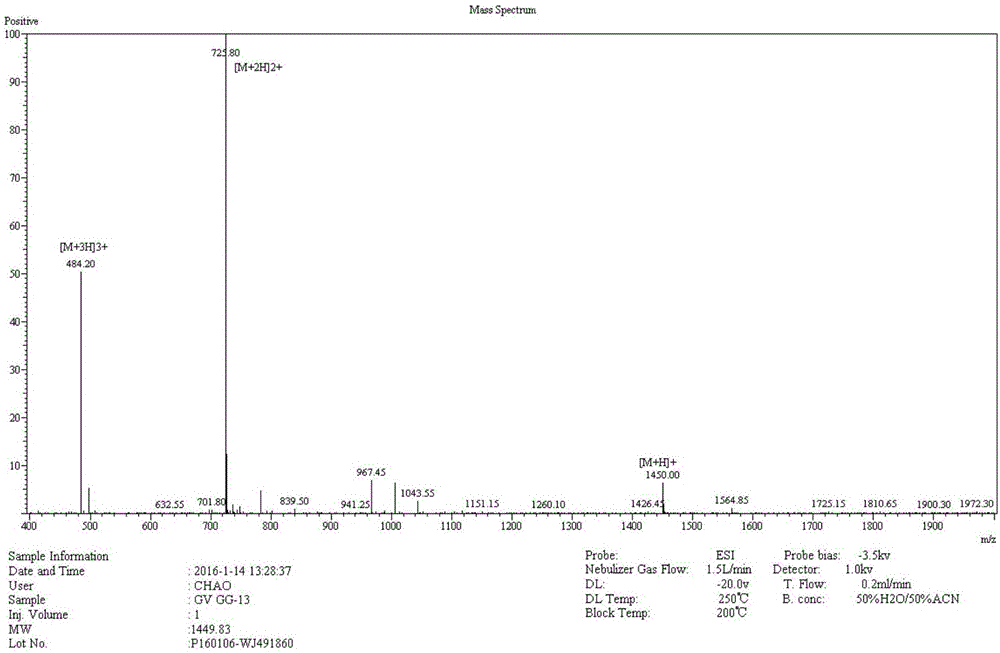
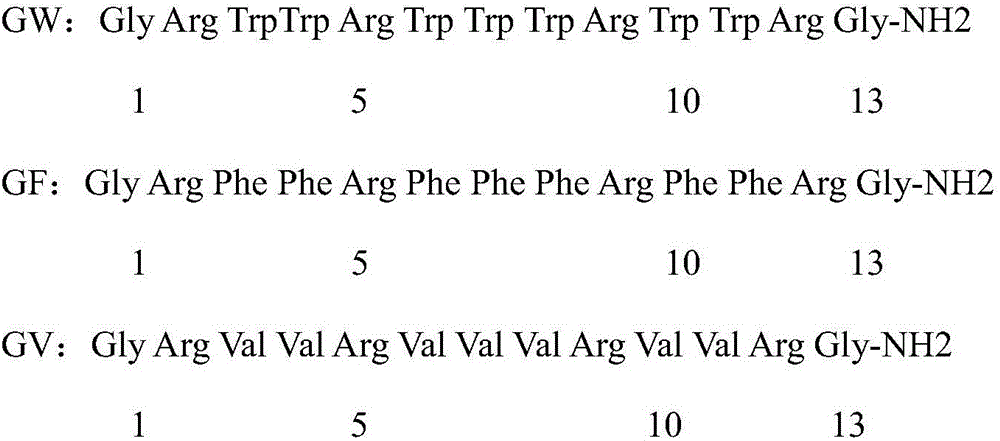
Antibacterial peptide GW13 and its preparation method and use
ActiveCN102827255ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsBeta defensinCombinatorial chemistry
The invention relates to an antibacterial peptide GW13 and its preparation method and use. The antibacterial peptide GW13 has an amino acid sequence of GlyLeuArgProLysTyrSer(ArgTrpLeu)2-NH2. The preparation method comprises the following steps of 1, cutting out 13 amino acid fragments from a linear chicken beta-defensin 4 carboxyl end by a fixed-point amino acid fragment cutting method, removing disulfide bonds to obtain a GW10 (GlyLeuArgProLysTyrSerArgTrpLeu-NH2) fragment, and repeating connecting characteristic three-residue complexes ArgTrpLeu to obtain the antibacterial peptide GW13, and 2, synthesizing a peptide resin in a polypeptide synthesizer by a solid-phase synthesis method, carrying out TFA cutting to obtain a polypeptide, and carrying out reversed-phase high-performance liquid chromatography purification. The antibacterial peptide GW13 obtained by the preparation method has strong bacteriostatic activity, low hemolytic activity, the highest therapeutic index and a large development potential.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Efficient alpha-helix antibacterial peptide GV and preparation method and application thereof
InactiveCN106366162ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsChemical synthesisAlpha helix
The invention relates to an efficient alpha-helix antibacterial peptide GV and a preparation method and application thereof. The antibacterial peptide GV is an antibacterial peptide with high cell selectivity and achieves an optimal point of junction of the antibacterial activity and cell toxicity of the antibacterial peptide. A sequence of the antibacterial peptide GV is represented by a sequence table SEQ ID No. 1. The preparation method comprises the following steps: (1) carrying out designing in accordance with alpha-helix peptide GRX2RX3RX2RG serves as a template, so as to obtain a brand-new antibacterial peptide GV, wherein X= V; (2) synthesizing a polypeptide crude product through a polypeptide synthesizer by a solid-phase chemical synthesis method; purifying the synthesized polypeptide by using out-phase high-performance liquid chromatography, and carrying out identification on the synthesized polypeptide by using electrospray mass spectrography, thereby preparing the polypeptide. The antibacterial peptide GV provided by the invention has relatively high bacteriostatic activity and relatively low hemolytic activity and is the highest in therapeutic index, thereby having great development potential.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
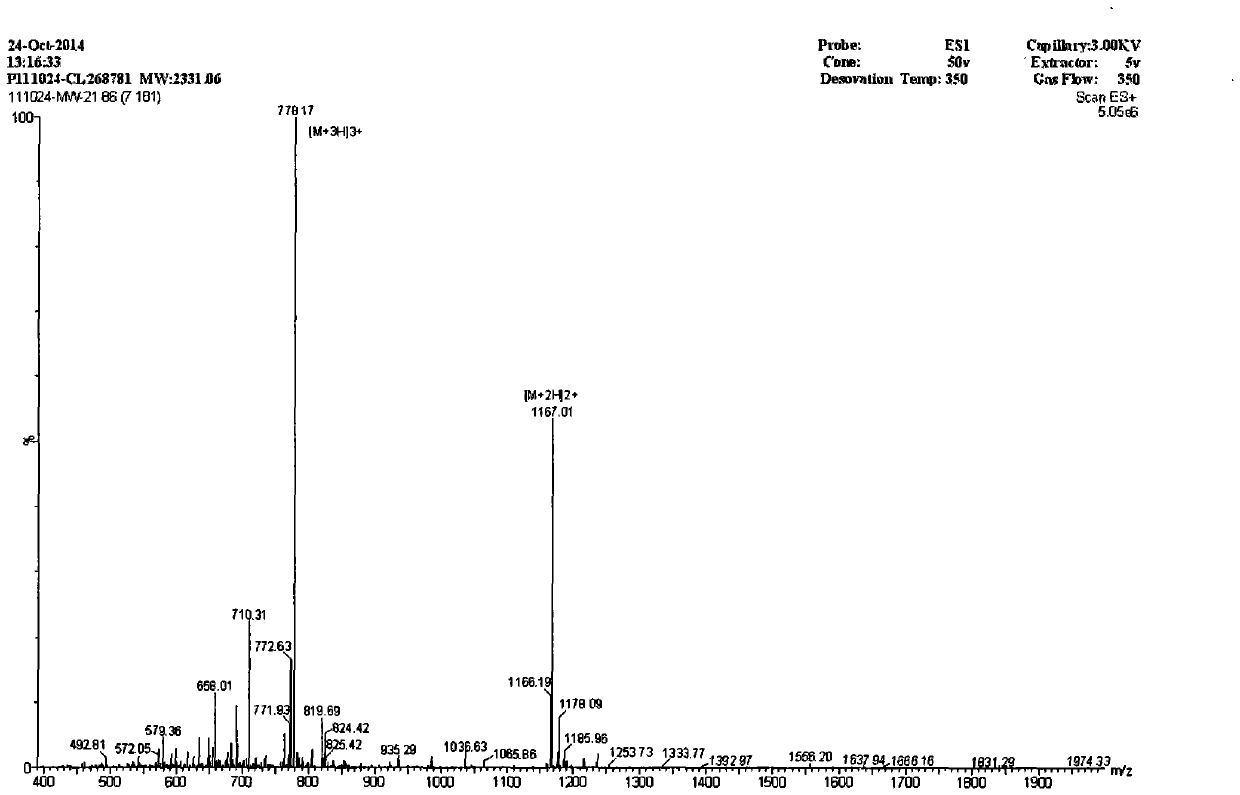
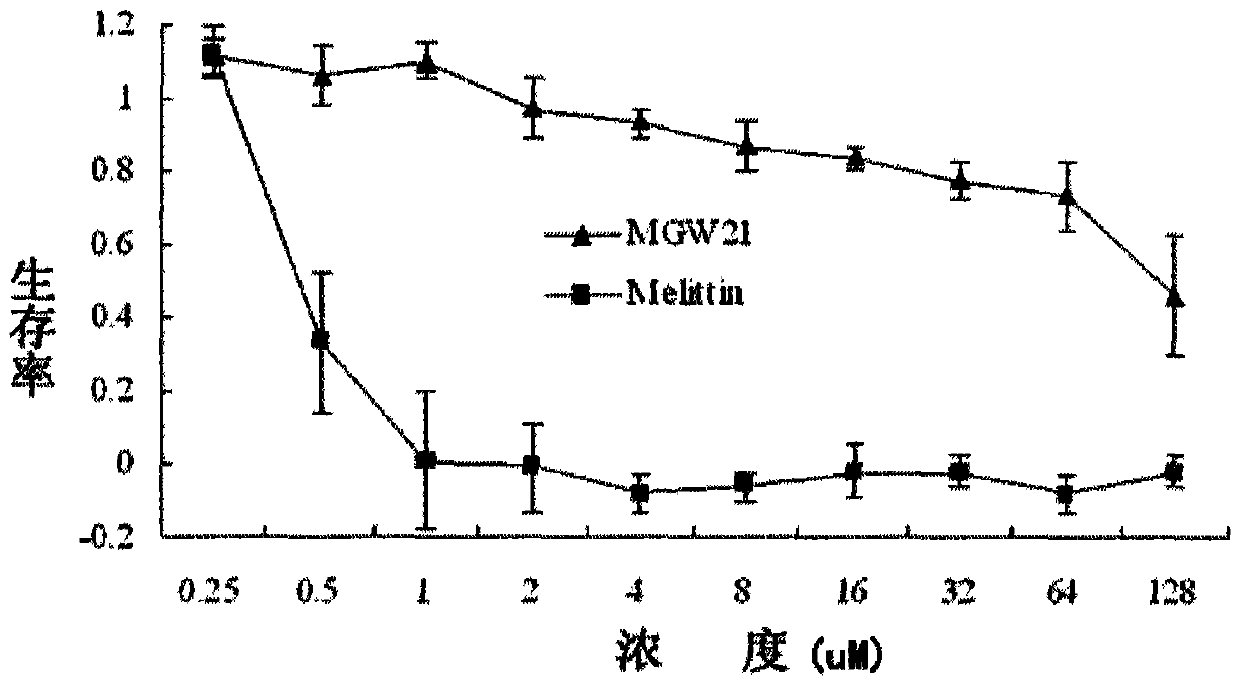
Derived peptide for chicken origin antibacterial peptide as well as preparation method and application thereof
InactiveCN104650208ASimple experimental techniqueGood inhibitory effectAntibacterial agentsPeptide/protein ingredientsChemical synthesisDisease
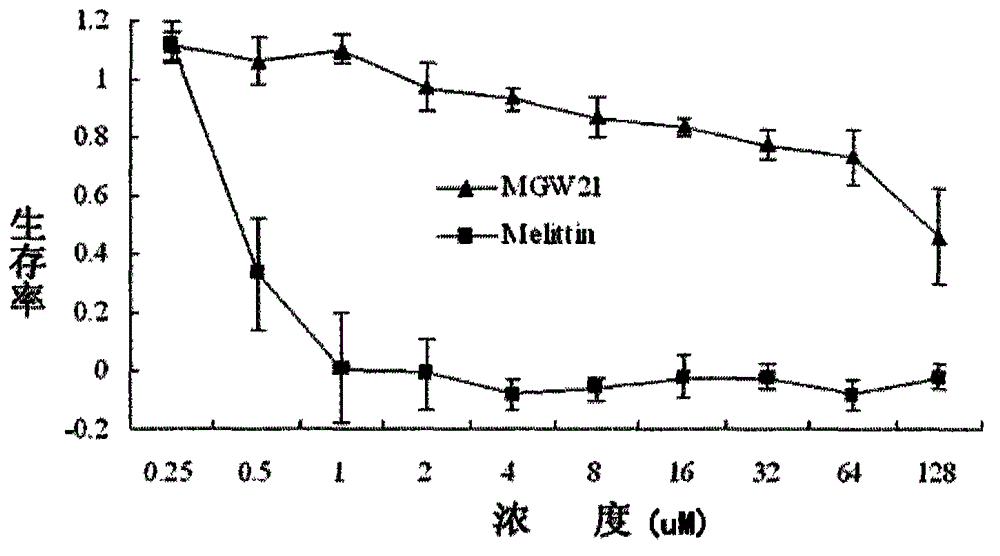
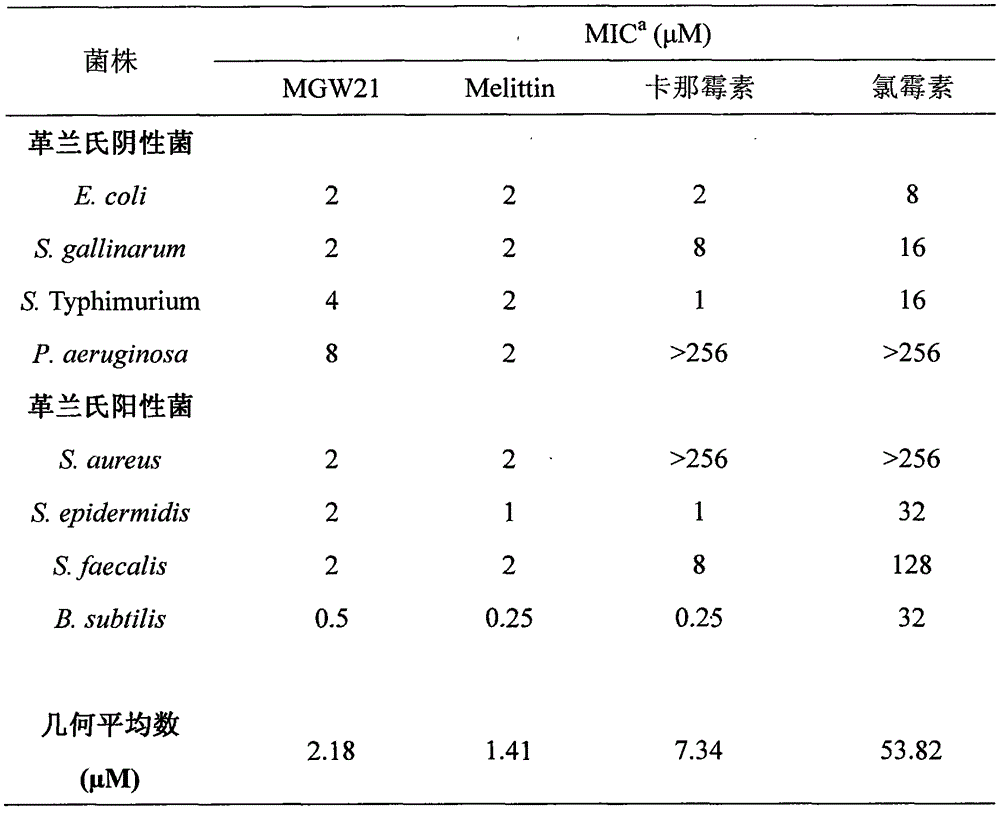
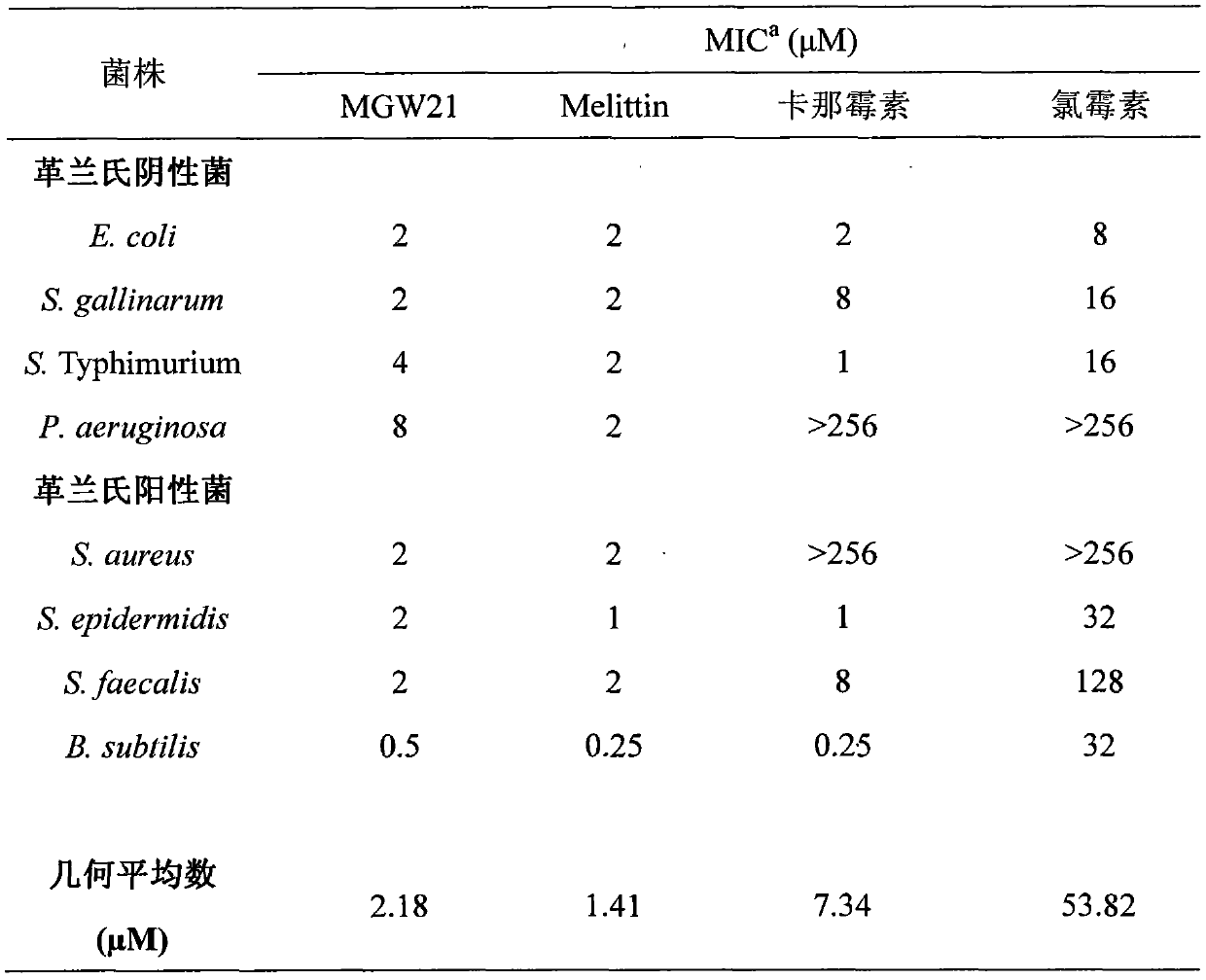
The invention provides a derived peptide for a chicken origin antibacterial peptide as well as a preparation method and application thereof. The sequence of the derived peptide is shown as SEQ No.1. The preparation method comprises the following steps: intercepting 20 amino acids of a chicken origin AvBD4 leader peptide, replacing 5th cysteine and 6th, 7th and 12th phenylalanine by amino acid lysine with charges respectively, meanwhile, carrying out amidation on a carboxyl terminal of the antibacterial peptide, and increasing a positive charge to improve the stability of the antibacterial peptide; adopting a solid-phase chemical synthesis method to obtain crude MGW21; and purifying and identifying synthesized polypeptide by using reversed-phase high performance liquid chromatography and electrospray mass spectrometry. The invention provides the application of the antibacterial peptide in preparing a medicament for treating gram-positive bacteria or gram-negative bacteria infectious diseases. The antibacterial peptide is good in amphiphilic property; compared with an antibiotic and Melittin, the antibacterial peptide is high in antibacterial activity and low in cytotoxicity.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Imperfect amphiphilic antimicrobial peptide PRW4-R and preparation method and application thereof
InactiveCN106554400ASimple experimental techniqueImprove cell selectivityAntibacterial agentsPeptide/protein ingredientsCrystallographyArginine
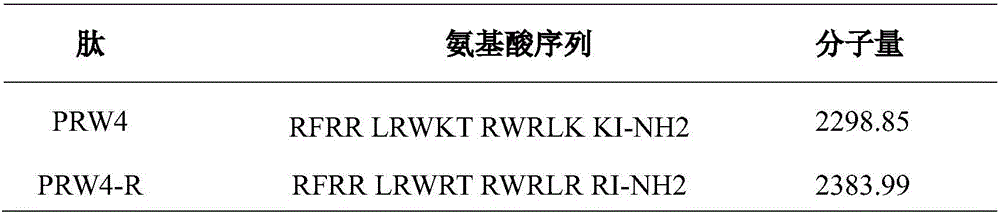
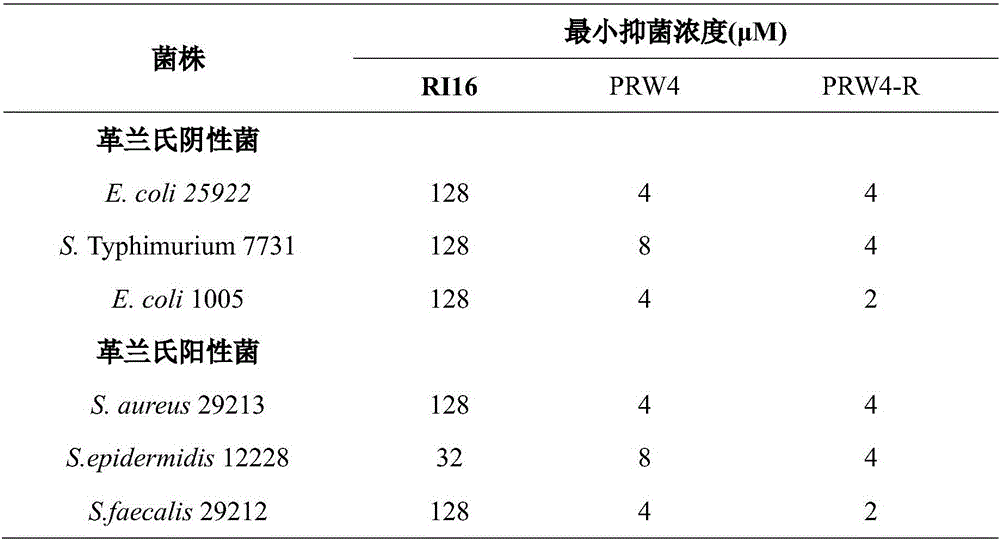
The invention provides an imperfect amphiphilic antimicrobial peptide PRW4-R and a preparation method and application thereof. The sequence of the imperfect amphiphilic antimicrobial peptide PRW4-R is shown as SEQ ID No. 1. The preparation method comprises the following steps: cutting out 16 amino acid sequences at a PMAP-36N end to obtain RI16 with a perfect amphiphilic structure; based on an Alpha-helix protein folding principle, substituting Trp for paired Lys in the polar face of the amphiphilic structure to obtain imperfect amphiphilic antimicrobial peptide PRW4; and substituting Arg for all lysine in the sequence of the PRW4 to obtain the PRW4-R. The therapeutic index of the PRW4-R is up to 161.5; and the peptide PRW4-R shows high cell selectivity and has low hemolytic activity, so that the development potential of the peptide PRW4-R in becoming a substituent of antibiotics is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Preparation method of spherical carbon-coated nickel with different carbon layers and hydrogen evolution property for water electrolysis of spherical carbon-coated nickel
InactiveCN110449157ASimple experimental techniqueEasy to operateMaterial nanotechnologyMetal/metal-oxides/metal-hydroxide catalystsNitrogen gasCarbon coated
The invention relates to the technical field of preparation of transition metal carbides, in particular to a preparation method of spherical carbon-coated nickel with different carbon layers and a hydrogen evolution property for water electrolysis of the spherical carbon-coated nickel. The preparation method comprises the following steps: (1) uniformly dispersing nickel nitrate hexahydrate and glycine in anhydrous ethanol by mechanical stirring; (2) transferring the mixed liquid to a reaction kettle, and carrying out a reaction for 12 hours at the temperature of 180 DEG C to obtain a carbon-coated nickel precursor; and (3) conducting roasting in nitrogen under a certain temperature condition to obtain the spherical carbon-coated nickel composite materials respectively. The method has the advantages that the raw materials are cheap and easy to obtain, synthesis is facilitated, equipment is simple, the production process is pollution-free, large-scale production can be quickly realized,and the material has lots of active sites and good hydrogen evolution properties for water electrolysis.
Owner:ZHENGZHOU UNIV
Enzymolysis-resisting antibacterial peptide I9H12 as well as preparation method and application thereof
ActiveCN109810178ASimple experimental techniqueHigh application potentialAntibacterial agentsAntimycoticsEnzyme digestionFungal Infectious Disease
The invention provides enzymolysis-resisting antibacterial peptide I9H12 as well as a preparation method and application thereof. A sequence of the antibacterial peptide I9H12 is shown as SEQ ID No. 1in a sequence table. The preparation method comprises the following steps: referring to the properties of amino acids; meanwhile, avoiding enzyme digestion sites of main proteinase in a body as muchas possible, and selecting hydrophobic amino acid isoleucine (Ile) and positive-charge amino acid histidine (His) as main amino acids to newly design the antibacterial peptide I9H12; carrying out amidation on a carboxyl terminal of the peptide to improve one positive charge and increase the stability of the peptide. The invention also provides the application of the antibacterial peptide to the preparation of targeting antibacterial medicines for treating gram-negative bacterium or fungus infectious diseases. According to the enzymolysis-resisting antibacterial peptide I9H12, the enzymolysis-resisting capability of the antibacterial peptide is effectively improved, and the application potential in actual production is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Targeting antibacterial peptide and preparation method and application thereof
ActiveCN106589135ASimple experimental techniquePrecise targeting specificityAntibacterial agentsPeptide/protein ingredientsAfter treatmentAntimicrobial peptides
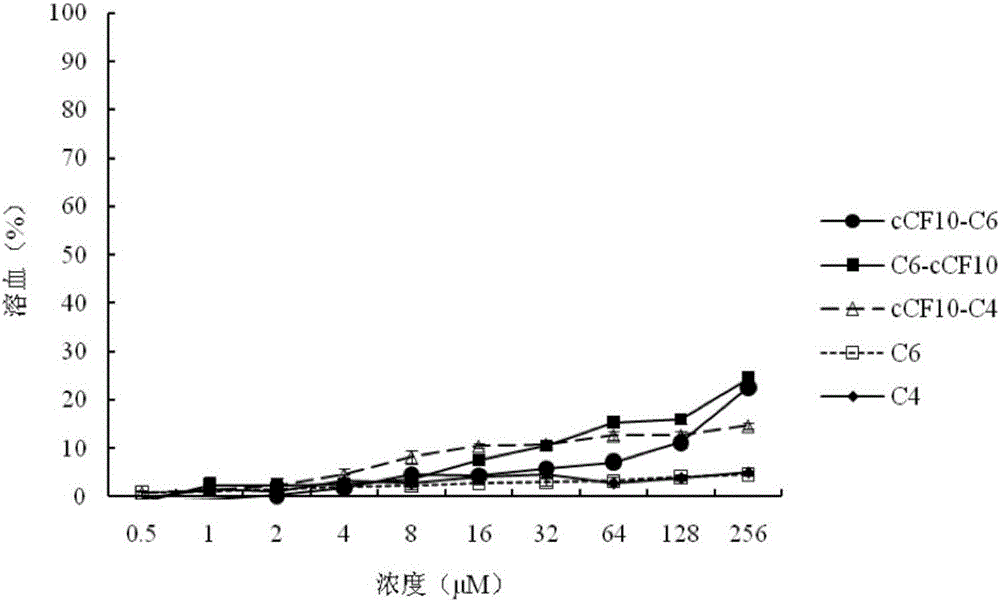
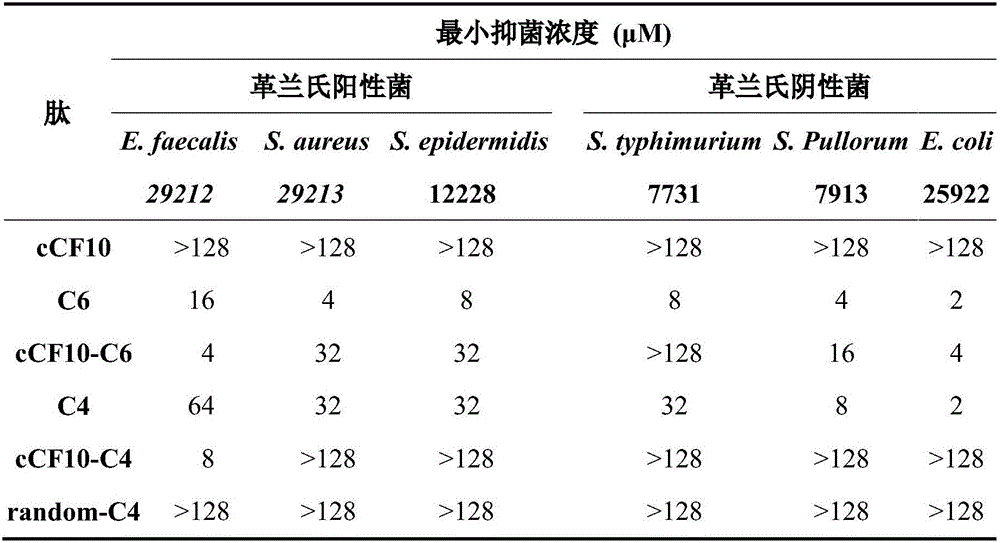
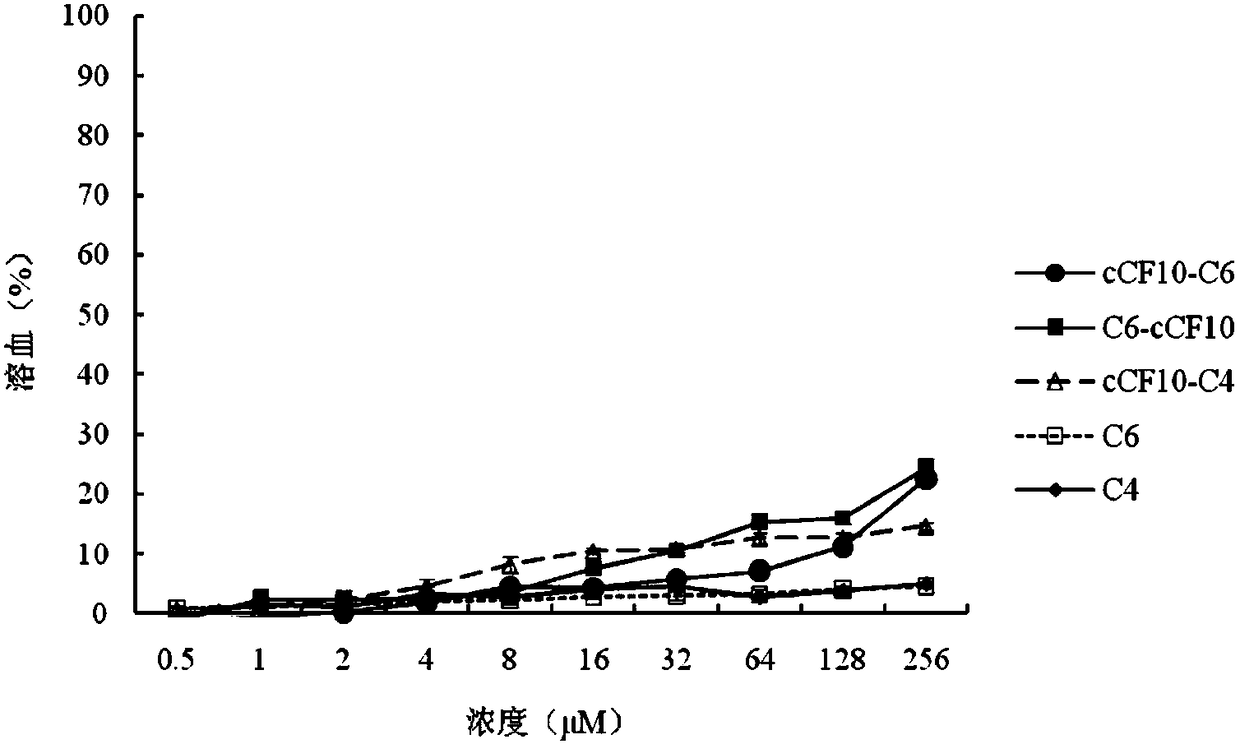
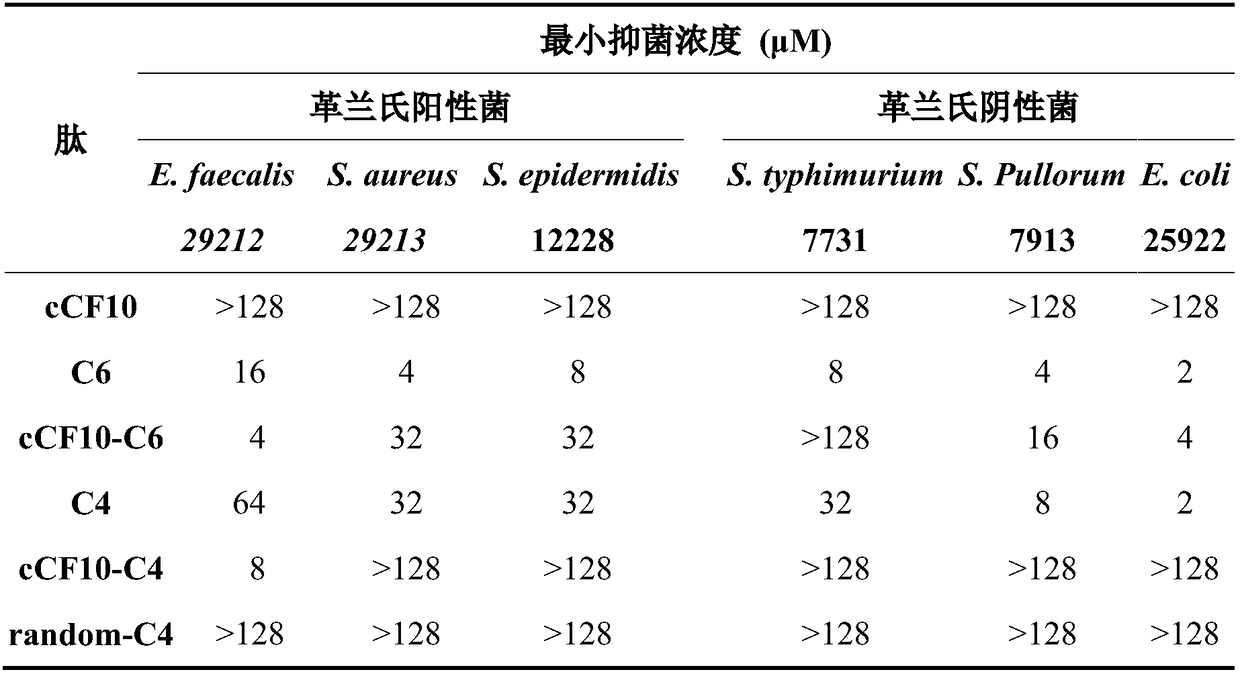
The invention discloses a targeting antimicrobial peptide and a preparation method and application thereof. A sequence of the targeting antimicrobial peptide cCF10-C4 is shown in a sequence table SEQ ID No.1. The preparation method comprises the steps that enterococcus faecalis serves as a target bacterium to obtain the targeting antimicrobial peptide cCF10-C4 through design, a series of multi-structural-domain antimicrobial peptide molecules having selectivity to the enterococcus faecalis are established in a heterozygous mode. The design of a series of antimicrobial peptide molecules mainly includes two independent functional areas, namely a sterilization area and an identification area respectively. The targeting antimicrobial peptide has targeting selection capability to the target bacterium. The targeting antimicrobial peptide cCF10-C4 is conductive to reestablishment of microecological balance after treatment.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Rapid detection kit for identifying SNP characteristic sites of Y chromosome haplotype pedigree and method
InactiveCN108531561AEasy to detectSimplify the experimental processMicrobiological testing/measurementFluorescenceMale individual
The invention discloses a kit for rapidly detecting the SNP site of a Y chromosome and a method, and the method is applied to the rapid identification of Y chromosome haplotype pedigrees of male individuals in forensic science. The rapid detection kit for the identification of the characteristic sites of the Y chromosome haplotype pedigrees of East Asian males includes primers corresponding to theSNP characteristic sites used for amplification detection of the Y chromosomes and probes of two mutation type sequence characteristics representing the SNP sites. The using method includes adding asample into plates having 96 or 384 pores, and performing an amplification reaction in a PCR instrument after a PCR reaction system is well mixed; putting the plates into the quantitative PCR instrument having an SDS scanning function to read a fluorescence signal after the amplification is finished; and automatically analyzing the Y-SNP haplotype of the sample by the instrument according to the fluorescence signal, and outputting a result in a spreadsheet. Through the kit, the detection can be completed by one step amplification, purification and template preparation are not needed, and the detection process of the Y-SNP can be simplified.
Owner:云南序源生物技术开发有限公司
Efficient hybrid antibacterial peptide LI and preparation method and application thereof
InactiveCN106432513ABroad spectrum biological activityHemolytic cytotoxicity is lowAntibacterial agentsPeptide/protein ingredientsAntibacterial activityCombinatorial chemistry
The invention relates to efficient hybrid antibacterial peptide LI and a preparation method and application thereof. A sequence of the efficient hybrid antibacterial peptide LI is shown as SEQ ID No.1 of a sequence table. The preparation method includes following steps: according to a fixed-point amino acid fragment intercepting method, intercepting an amino acid residue fragment from the 14th locus to the 21st locus of human-derived antibacterial peptide LL37 and an amino acid residue fragment from the 5th locus to the 13th locus of bovine-derived antibacterial Indolicidin, and sequentially connecting the two fragments to obtain the antibacterial peptide LI; 2), using a polypeptide synthesizer for solid-phase synthesis to synthesize peptide resin; obtaining polypeptide after TFA cutting, and adopting reverse-phase high-performance liquid chromatography for purifying. The antibacterial peptide LI obtained by the method has high antibacterial activity, weak hemolytic activity, high treatment index and huge development potential.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Preparation method and application of beta-phase ferronickel hydroxide/carbon nanotube compound with atomic defects
ActiveCN112044442AHarm reductionReduce pollutionMetal/metal-oxides/metal-hydroxide catalystsElectrodesHydration reactionMeth-
The invention discloses a preparation method and application of a beta-phase ferronickel hydroxide / carbon nanotube compound with atomic defects, and the preparation method comprises the following steps: (1) uniformly dispersing carbon oxide nanotubes in deionized water (introduced with nitrogen) by adopting an ultrasonic dispersion technology; (2) adding nickel chloride hexahydrate, ferrous chloride tetrahydrate, hexamethylenetetramine and ammonium fluoride into the solution, transferring into a reaction kettle, and reacting at 120 DEG C for 6 hours to obtain a ferronickel hydroxide / carbon nanotube compound; and (3) dispersing the obtained material in a mixed solution of hydrogen peroxide and water at room temperature to oxidize ferronickel hydroxide / carbon nanotube compounds with different defect degrees for different time. The method has the advantages of cheap and accessible raw materials, convenient synthesis, simple equipment and no pollution in the production process, can quicklyimplement large-scale production, and has the advantages of more defect sites, more active centers and favorable water electrolysis and oxygen evolution properties.
Owner:ZHENGZHOU UNIV
A kind of derivative peptide of chicken source antimicrobial peptide and its preparation method and application
InactiveCN104650208BSimple experimental techniqueEnhanced inhibitory effectAntibacterial agentsPeptide/protein ingredientsChemical synthesisAntimicrobial peptides
The invention provides a derived peptide for a chicken origin antibacterial peptide as well as a preparation method and application thereof. The sequence of the derived peptide is shown as SEQ No.1. The preparation method comprises the following steps: intercepting 20 amino acids of a chicken origin AvBD4 leader peptide, replacing 5th cysteine and 6th, 7th and 12th phenylalanine by amino acid lysine with charges respectively, meanwhile, carrying out amidation on a carboxyl terminal of the antibacterial peptide, and increasing a positive charge to improve the stability of the antibacterial peptide; adopting a solid-phase chemical synthesis method to obtain crude MGW21; and purifying and identifying synthesized polypeptide by using reversed-phase high performance liquid chromatography and electrospray mass spectrometry. The invention provides the application of the antibacterial peptide in preparing a medicament for treating gram-positive bacteria or gram-negative bacteria infectious diseases. The antibacterial peptide is good in amphiphilic property; compared with an antibiotic and Melittin, the antibacterial peptide is high in antibacterial activity and low in cytotoxicity.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Simple preparation method of hollow-spherical and flower-shaped indium oxide with secondary structure and application
InactiveCN102134092BWide variety of sourcesLow costGallium/indium/thallium compoundsChemiluminescene/bioluminescenceIndiumActive agent
The invention provides a preparation method of indium oxide with new appearance, belonging to the technical field of synthesis and nano material of the indium oxide. The preparation method comprises the following steps: taking inorganic salt solution of the indium as an indium source, adopting N, N-dimethyl formamide (DMF) as an alkali source and anion active agents such as sodium dodecyl sulfate (SDS) and the like as an additive, using hydrothermal synthesis to prepare the novel hollow-microspherical and flower-shaped indium oxide with the secondary structure under the condition without adding any hard template, and controlling the appearance of the secondary structure by chemical means. In the preparation method provided by the invention, the technical process is simple, the promotion is easy, the selected reagent is low in price and environment-friendly, and the need on low-cost, large-scale and green production is met.
Owner:SICHUAN UNIV
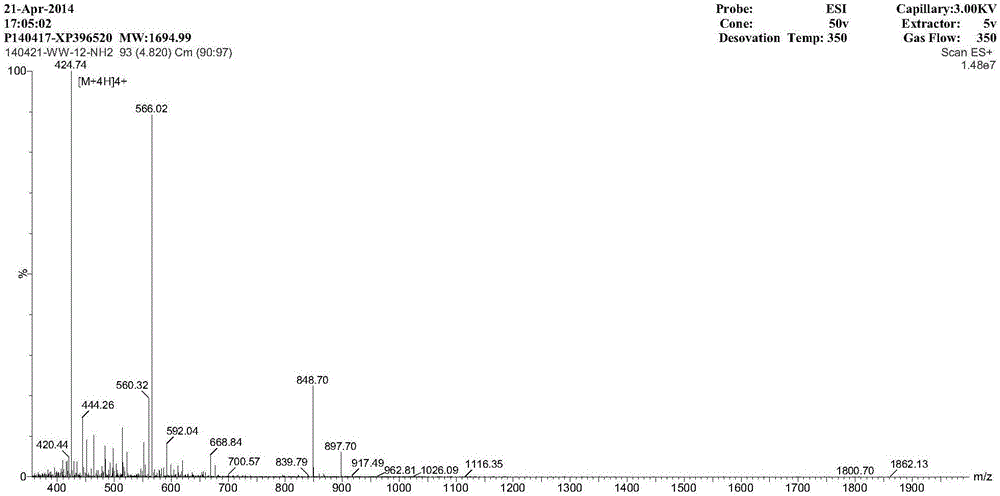
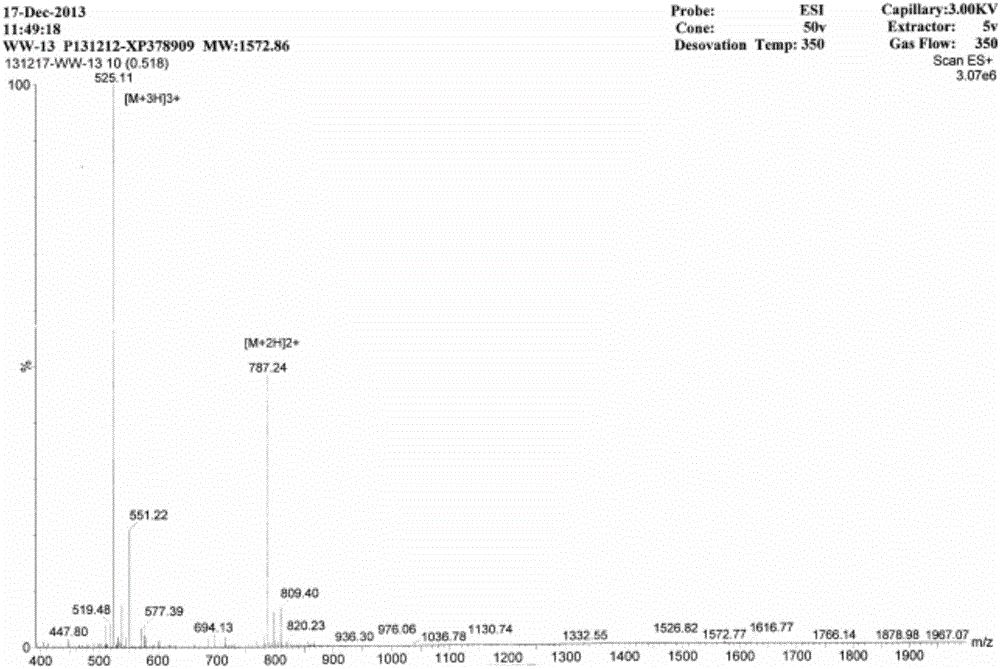
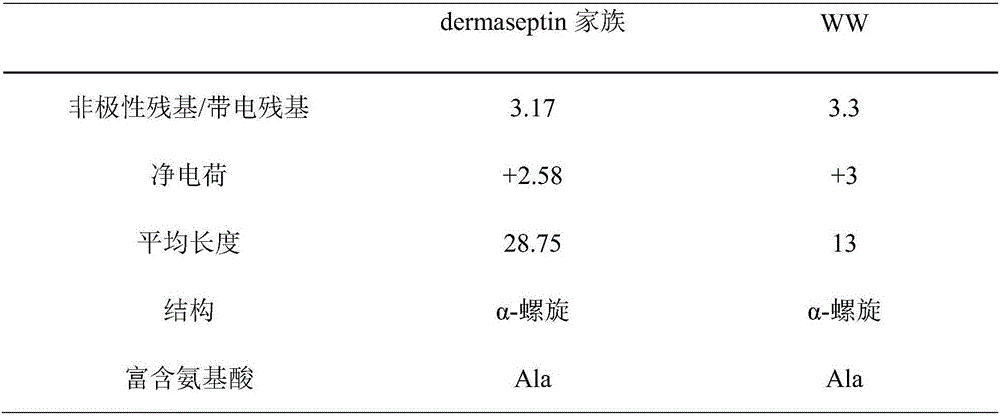
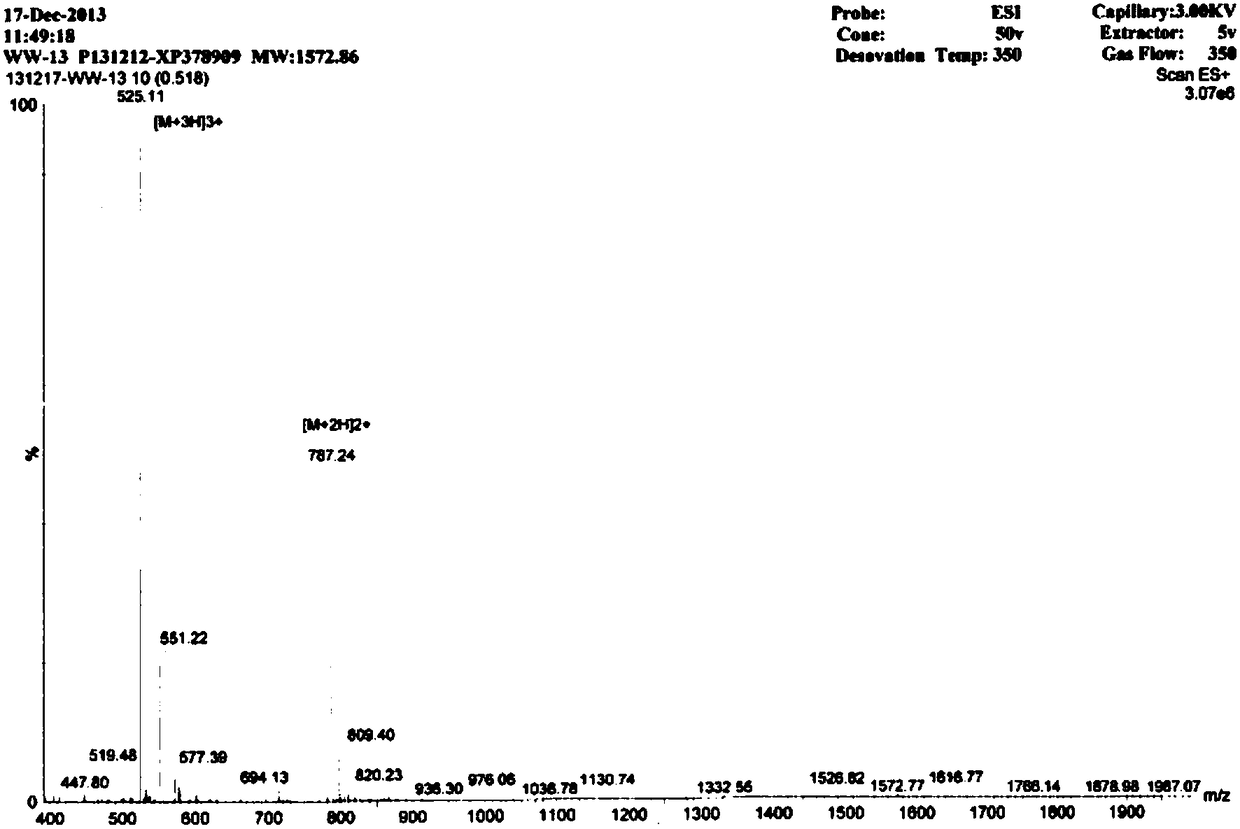
AMP (antimicrobial peptide) WW based on peptide miniaturization strategy as well as preparation method and application of AMP WW
ActiveCN106518999ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsDermaseptinMiniaturization
The invention provides AMP (antimicrobial peptide) WW based on a peptide miniaturization strategy as well as a preparation method and an application of the AMP WW. The sequence of the AMP WW is shown as SEQ ID NO.1 in the sequence table. By adopting the peptide miniaturization theory and a bioactivity improving strategy and taking dermaseptin-family peptides secreted by amphibian frog skin as a template, the sequence containing key physical parameters of mother peptides and having the length shortened to 13 amino acids is obtained. The bacteriostatic activity of WW is slightly lower than that of melittin, while the therapeutic index is as high as 69.8 and is 698 times that of the melittin. With adoption of the method, risks of immunogenicity and heterogenous repellency are avoided theoretically, the selectivity of the AMP between bacterial cells and mammalian cells is improved, and the development potential of the AMP for becoming an antibiotic substitute is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Internal reference gene and construction method for PCR expression analysis of Caucasian clover
ActiveCN109136403BSimple experimental techniqueImprove stabilityMicrobiological testing/measurementFermentationReference genesNucleotide sequencing
The invention discloses an internal reference gene and a construction method for PCR (polymerase chain reaction) expression analysis of caucasian clover. A nucleotide sequence of the internal reference gene is as shown in the sequence SEQ ID No.2; the construction method comprises the steps as follows: selecting 7 internal reference genes with stable expression by utilizing 7 tissue transcriptomedata of caucasian clover, and selecting one traditional internal reference gene commonly used in clover as a control; performing primer design on the candidate internal reference genes, performing PCRamplification on each cDNA, and obtaining the amplification efficiency of primers and the cycle number of amplification reaching a threshold value; evaluating and analyzing the obtained data for internal reference stability, and finally screening the most suitable internal reference gene and the number of the internal reference gene under specific experimental conditions. The reference gene is used as a reference for detecting the gene expression level change of caucasian clover, and for correcting the loading quantity of sample and an experimental error existing in a sample loading process,so as to ensure the accuracy of experimental results.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
A targeted antibacterial peptide for Gram-negative bacteria, its production method and application
ActiveCN109232717BSimple experimental techniqueHigh application potentialAntibacterial agentsPeptide/protein ingredientsBiotechnologyAntimicrobial peptides
The invention provides a targeted antimicrobial peptide for Gram-negative bacteria, a production method and application thereof. Its sequence is shown in the sequence table SEQ ID No.1. The preparation method uses the R language to statistically analyze the amino acid sequence characteristics of the natural antibacterial peptide library, and for the peptide chains that only have antibacterial activity against Gram-negative bacteria, the peptides that affect the antibacterial activity The chain parameters were statistically analyzed, and the optimal amino acid composition was screened, namely K, G, L; the number of positive charges: 4, and the hydrophobicity: 30%-50%. Insert tryptophan into the center of the screening sequence to design a centrosymmetric short peptide sequence; to obtain a short-chain narrow-spectrum antimicrobial peptide sequence with low toxicity, high efficiency, and less likely to cause immune regulation disorders in the body. The targeted antimicrobial peptide is beneficial to kill harmful bacteria while maintaining micro-ecological balance. The antimicrobial peptide has high activity, strong stability, and relatively low cytotoxicity. It has the potential to replace antibiotics and become a safer and environmentally friendly new antibacterial product. .
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
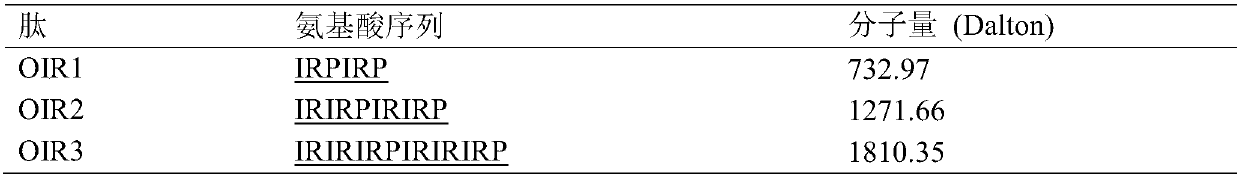
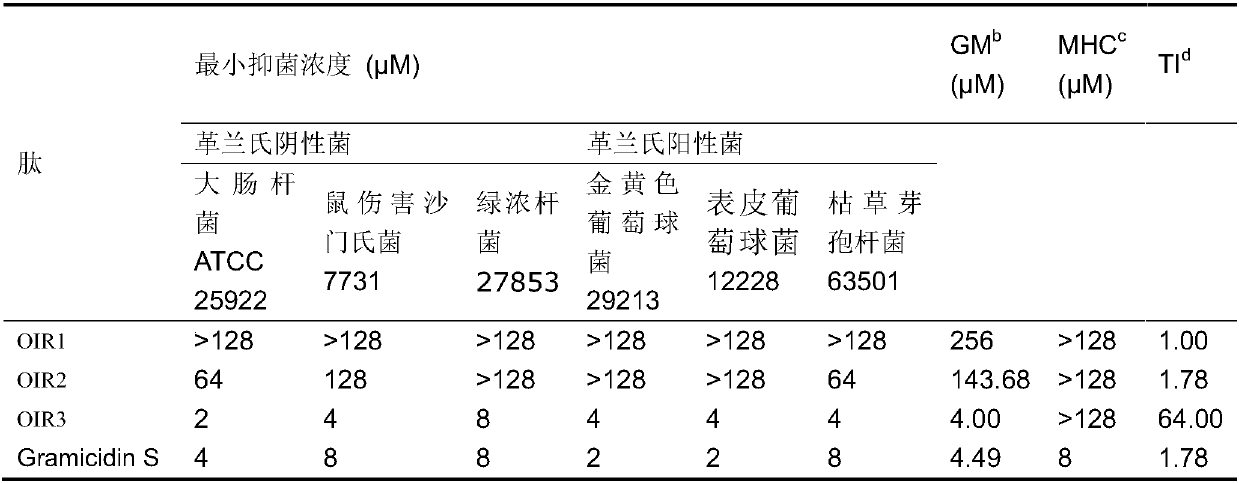
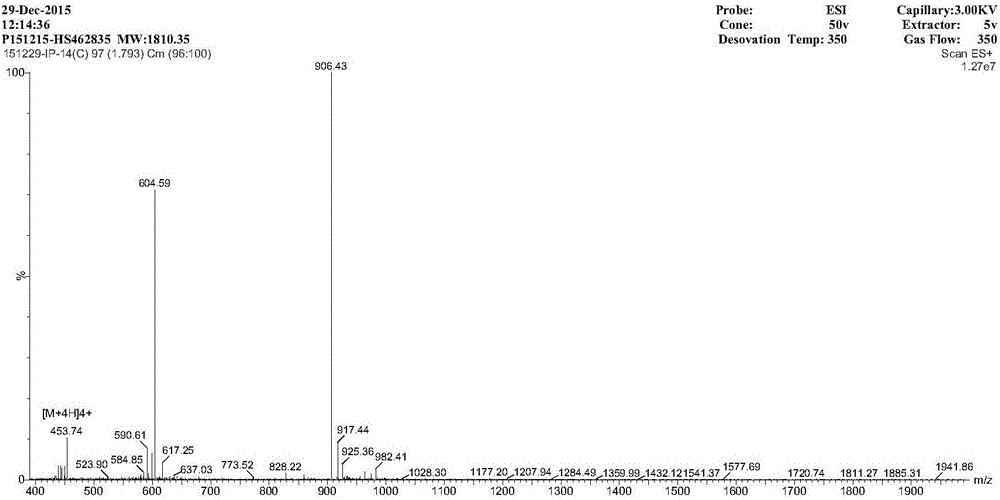
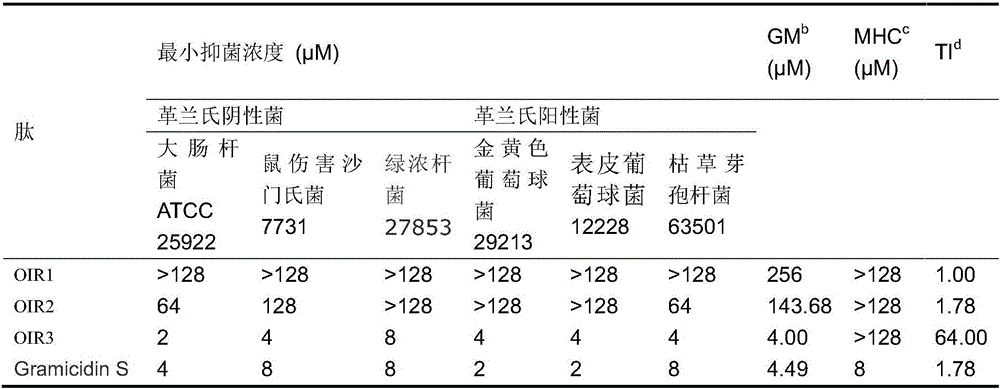
Cyclic antimicrobial peptide oir3 with high cell selectivity and its preparation method and application
InactiveCN106749544BSimple experimental techniqueEnhanced inhibitory effectAntibacterial agentsPeptidesHigh cellDisease
The invention provides a high cell-selectivity cyclic antimicrobial peptide OIR3 and a preparation method and application thereof. The high cell-selectivity cyclic antimicrobial peptide OIR3 has a sequence shown in the formula of SEQ ID No. 1 in the sequence table. The preparation method comprises 1) designing a cyclic antimicrobial peptide OIR3 according to a cyclic antimicrobial peptide template (IleArg)nPro(IleArg)n, wherein n is 3, and 2) synthesizing a peptide resin by a solid phase synthesis method using a polypeptide synthesizer, carrying out TFA cutting to obtain a polypeptide, and carrying out reversed-phase high-performance liquid chromatography purification so that the antimicrobial peptide OIR3 is obtained. The antimicrobial peptide can be used in drugs for treating diseases infected by gram-positive bacteria or gram-negative bacteria. The antimicrobial peptide OIR3 is a high cell-selectivity cyclic antimicrobial peptide, has strong antibacterial activity and weak hemolytic activity, has the highest treatment index and has a great development potential.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
High cell-selectivity cyclic antimicrobial peptide OIR3 and preparation method and application thereof
InactiveCN106749544ASimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptidesHigh cellDisease
The invention provides a high cell-selectivity cyclic antimicrobial peptide OIR3 and a preparation method and application thereof. The high cell-selectivity cyclic antimicrobial peptide OIR3 has a sequence shown in the formula of SEQ ID No. 1 in the sequence table. The preparation method comprises 1) designing a cyclic antimicrobial peptide OIR3 according to a cyclic antimicrobial peptide template (IleArg)nPro(IleArg)n, wherein n is 3, and 2) synthesizing a peptide resin by a solid phase synthesis method using a polypeptide synthesizer, carrying out TFA cutting to obtain a polypeptide, and carrying out reversed-phase high-performance liquid chromatography purification so that the antimicrobial peptide OIR3 is obtained. The antimicrobial peptide can be used in drugs for treating diseases infected by gram-positive bacteria or gram-negative bacteria. The antimicrobial peptide OIR3 is a high cell-selectivity cyclic antimicrobial peptide, has strong antibacterial activity and weak hemolytic activity, has the highest treatment index and has a great development potential.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
A kind of targeting antimicrobial peptide and its preparation method and application
ActiveCN106589135BSimple experimental techniquePrecise targeting specificityAntibacterial agentsPeptide/protein ingredientsAfter treatmentAntimicrobial peptides
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
A kind of enzymolysis-resistant antimicrobial peptide i9h12 and its preparation method and application
ActiveCN109810178BSimple experimental techniqueHigh application potentialAntibacterial agentsAntimycoticsDiseaseCarboxyl radical
The invention provides an anti-enzymatic antibacterial peptide I9H12 and a preparation method and application thereof. The sequence of antimicrobial peptide I9H12 is shown in SEQ ID No. 1 of the sequence listing. Preparation method: Referring to the properties of amino acids, while avoiding the cleavage sites of major proteases in the body as much as possible, the hydrophobic amino acid isoleucine (Ile) and the positively charged amino acid histidine (His) were selected as the main amino acids to design a new I9H12 antimicrobial peptide , and amidation of the carboxy terminus of the peptide to increase a positive charge and increase the stability of the peptide. Application of the antibacterial peptide in the preparation of targeted antibacterial drugs for treating Gram-negative bacteria or fungal infectious diseases. The invention effectively improves the anti-enzymatic hydrolysis ability of the antibacterial peptide and improves its application potential in actual production.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Derivative peptide w8 based on amphibian frog-derived antimicrobial peptide and its preparation method and application
ActiveCN109553677BSimple experimental techniqueHigh clinical application valueAntibacterial agentsAntimycoticsDiseaseAntimicrobial peptides
The present invention provides a derivative peptide W8 based on amphibian frog-derived antimicrobial peptide and its preparation method and application. The sequence of the derivative peptide W8 is shown in SEQ ID No.1. The preparation method is as follows: According to the amphibian frog-derived antimicrobial peptide, the antimicrobial peptide P8 was designed. Its sequence is as follows: AARILRPRFR. As follows: AARIILRWRFR. The application of the derived peptide W8 in the preparation of medicines for treating Gram-negative bacteria, Gram-positive bacteria and fungal infectious diseases. The antibacterial activity of antibacterial peptide W8 is significantly stronger than that of Kunitzin‑RE, and it has a significant broad-spectrum antibacterial activity that Kunitzin‑RE does not possess. W8 improves the selectivity of antimicrobial peptides between bacterial cells and mammalian cells.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
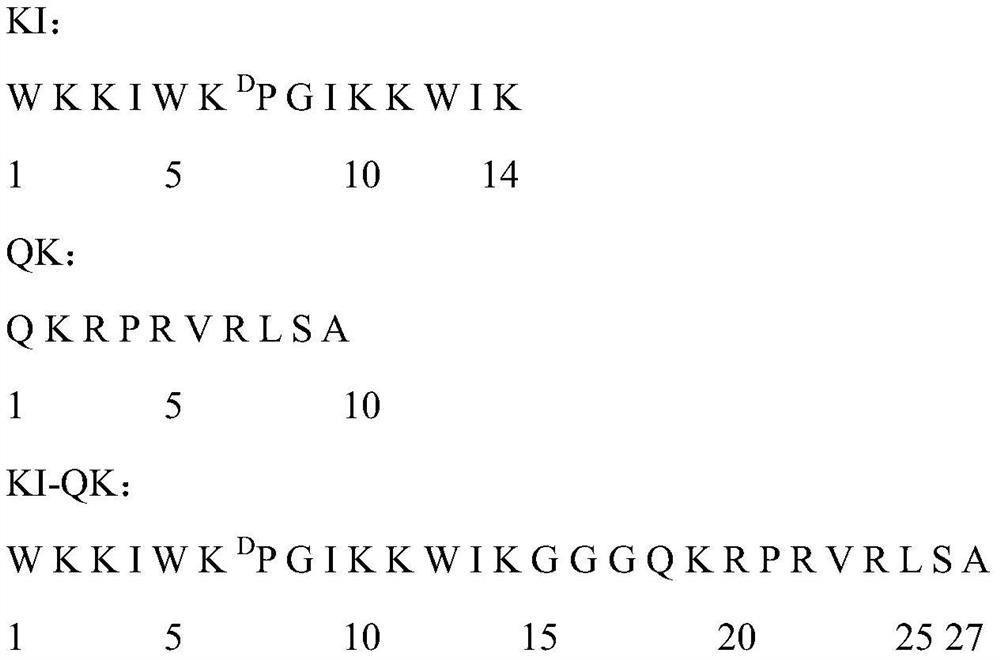
A kind of Escherichia coli targeting antimicrobial peptide ki-qk and its preparation method and application
ActiveCN109705195BSimple experimental techniqueHigh antibacterial activityAntibacterial agentsPeptide/protein ingredientsEscherichia coliGlycine
The present invention provides a method for designing Escherichia coli targeting antimicrobial peptide KI-QK, the sequence of which is shown in SEQ ID No.1 in the sequence table. A peptide KI with an α-helical structure is used, and peptide QK is connected to its C-terminus. At the same time, three flexible amino acids glycine are used as linkers between KI and QK, and the peptide is named KI-QK. In the biological activity test, we found that KI‑QK has a very strong antibacterial activity against Escherichia coli, and its geometric mean minimum inhibitory concentration against Escherichia coli is 3.667, which is 4.2 times higher than that of the original peptide KI. The index is 69.812, which is 4.7 times higher than that of the original peptide. In summary, KI‑QK is a typical targeting antimicrobial peptide, which has strong targeting effect on Escherichia coli and has high application value.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
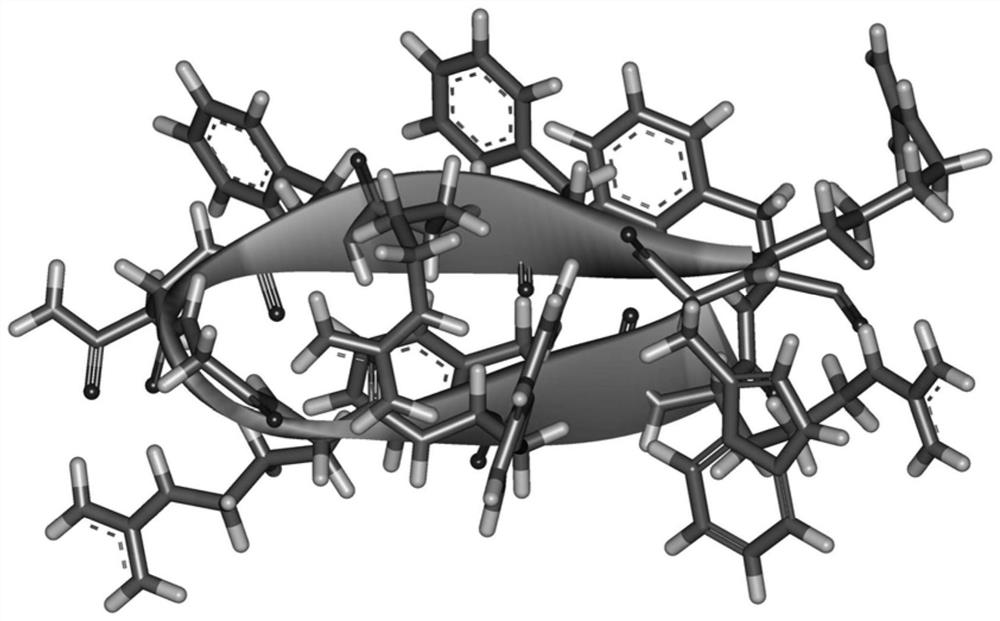
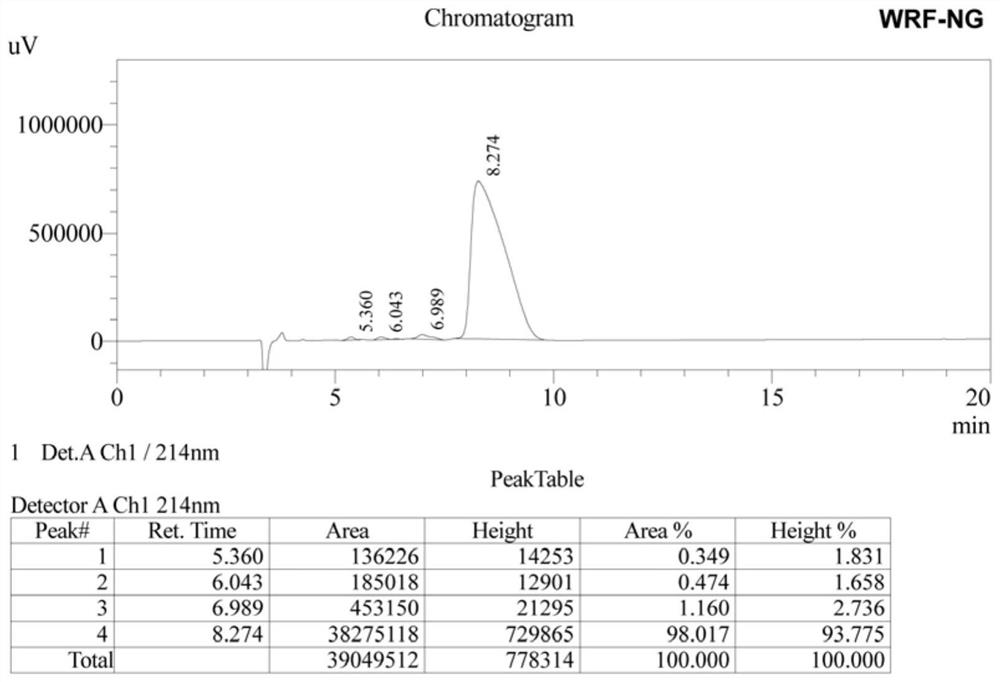
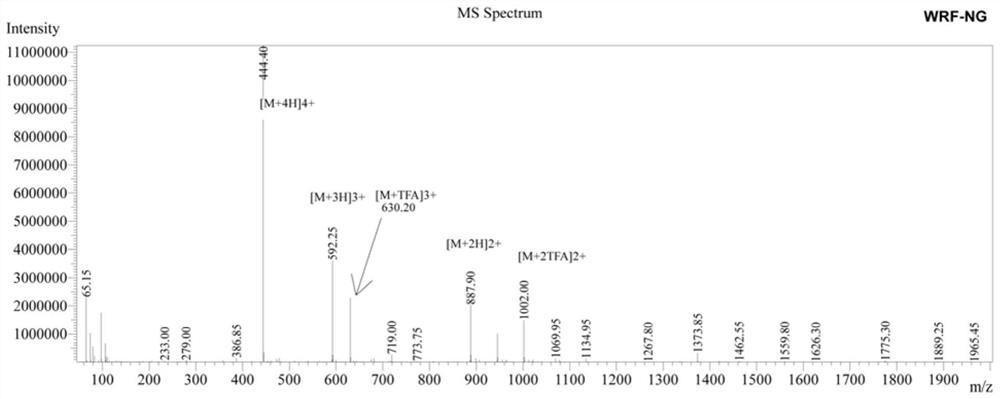
β hairpin antimicrobial peptide containing asparagine and glycine turn angle and preparation method thereof
ActiveCN111484546BShort sequence lengthStable structureAntibacterial agentsPeptide/protein ingredientsGlycineAssay
The invention provides a β-hairpin antibacterial peptide containing asparagine and glycine turn angle and a preparation method. The sequence of the antimicrobial peptide WRFNG of the present invention is shown in SEQ ID No.1. The preparation method of the present invention uses the Asn-Gly corner as the corner unit, forms the mutual attraction of two β-side chains with the interaction of Trp and Arg, and designs the antibacterial peptide template XWYRYZZXWYRY-NH according to the arrangement principle of the β-hairpin structure 2 . X=R, Y=F, ZZ=NG. The application of the antibacterial peptide in the preparation of drugs for treating infectious diseases caused by Gram-positive bacteria and / or Gram-negative bacteria. The therapeutic index of the antimicrobial peptide of the present invention reaches 138.30, which is 1.58 times higher than WRFPG with Pro-Gly corner, and 3.55 times higher than WRFpG with D-Pro-Gly corner, and has the development potential to become a green and efficient antibiotic substitute.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Antimicrobial peptide ww based on micropeptide strategy and its preparation method and application
ActiveCN106518999BSimple experimental techniqueLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsDermaseptinMiniaturization
The invention provides AMP (antimicrobial peptide) WW based on a peptide miniaturization strategy as well as a preparation method and an application of the AMP WW. The sequence of the AMP WW is shown as SEQ ID NO.1 in the sequence table. By adopting the peptide miniaturization theory and a bioactivity improving strategy and taking dermaseptin-family peptides secreted by amphibian frog skin as a template, the sequence containing key physical parameters of mother peptides and having the length shortened to 13 amino acids is obtained. The bacteriostatic activity of WW is slightly lower than that of melittin, while the therapeutic index is as high as 69.8 and is 698 times that of the melittin. With adoption of the method, risks of immunogenicity and heterogenous repellency are avoided theoretically, the selectivity of the AMP between bacterial cells and mammalian cells is improved, and the development potential of the AMP for becoming an antibiotic substitute is improved.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com