Efficient hybrid antibacterial peptide LI and preparation method and application thereof
An antimicrobial peptide and high-efficiency technology, which is applied in the field of high-efficiency hybrid antimicrobial peptide LI and its preparation and application, can solve the problems of small molecular weight, low activity and complex structure of antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
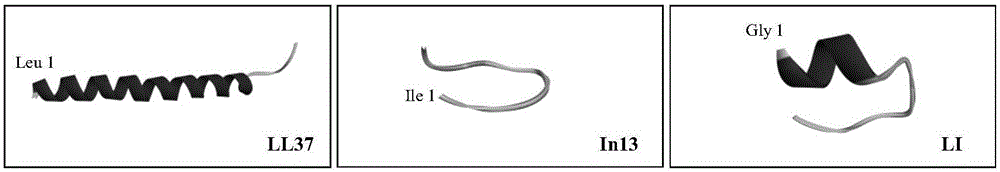
[0016] The amino acid sequence of human antimicrobial peptide LL37 is:
[0017]
[0018] The amino acid sequence of bovine antimicrobial peptide In13 is:
[0019] Ile Leu Pro Trp Lys Trp Pro Trp Trp Pro Trp Arg Arg-NH2
[0020] 1 5 10 13
[0021] According to the site-specific amino acid fragment interception method, the amino acid residues 14-21 of human antimicrobial peptide LL37 (GlyLysGluPheLysArgIleVal) and the fragments of amino acid residues 5-13 of bovine antimicrobial peptide In13 (LysTrpProTrpTrpProTrpArgArg) were intercepted.
[0022] The above two fragments were sequentially connected to obtain LI (GlyLysGluPheLysArgIleValLysTrpProTrpTrpProTrpArgArg-NH 2 ) antimicrobial peptides.
Embodiment 2
[0024] The above two antimicrobial peptides were synthesized using a peptide synthesizer. The method was solid-phase chemical synthesis, and the specific steps were:
[0025] 1) The preparation of antimicrobial peptides is carried out one by one from the C-terminus to the N-terminus, and is completed by a peptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminal of each antimicrobial peptide) is inserted into Wang resin, and then the Fmoc group is removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fmoxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antimicrobial peptide); according to this procedure, it is synthesized from the C-terminus to the N-terminus until the synthesis is completed, and the side of the Fmoc group is removed chain protection resin;
[0026] Add cutting reagent to the peptide resin obtained above, react at 20°C for 2 hours in the dark, filter; precipitate TFA (trifluoroacetic acid) for washing, mix the washing...
Embodiment 3
[0032] The designed and synthesized antimicrobial peptides were compared and detected by in vitro antibacterial and hemolytic activity tests;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com