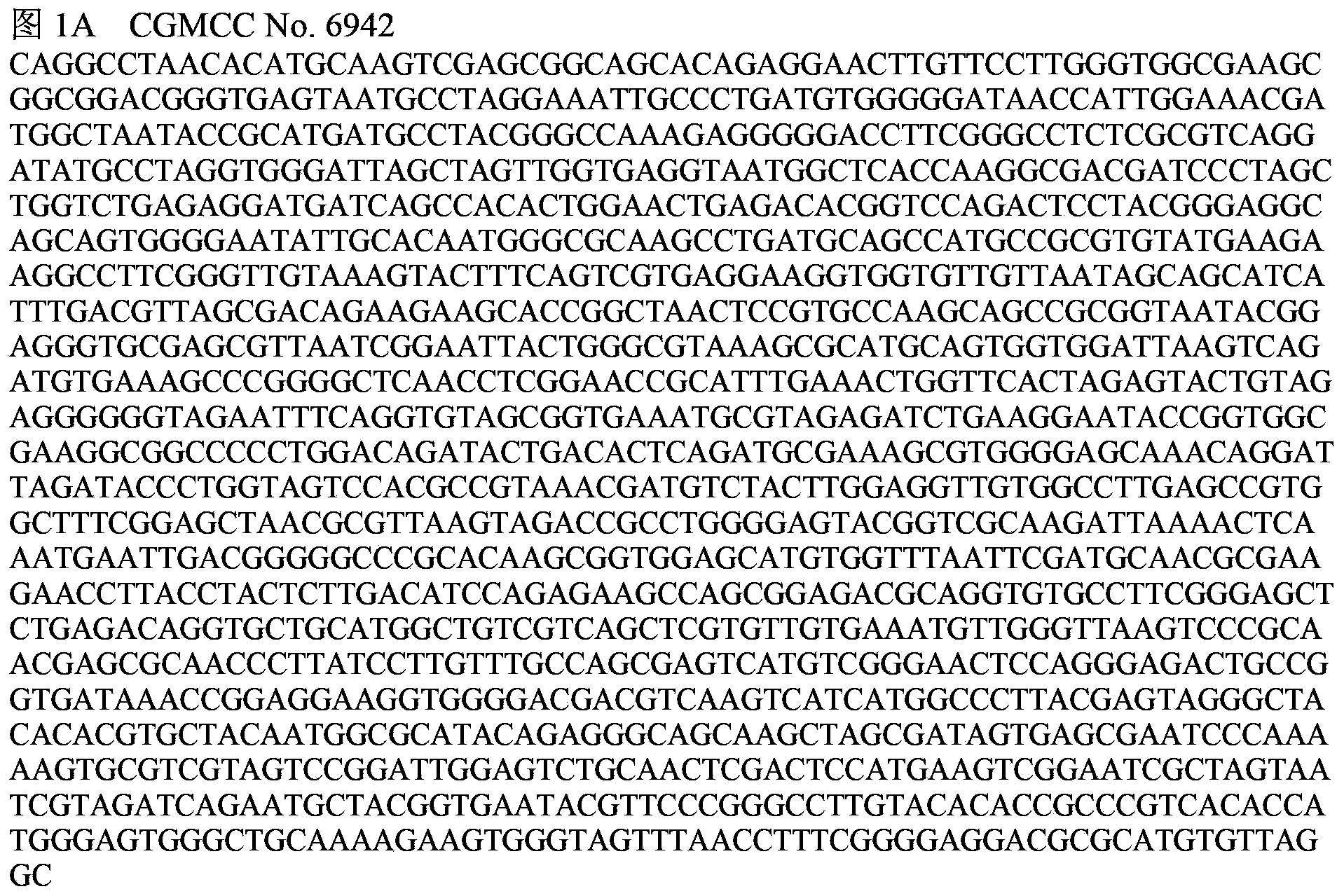Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
73results about How to "Effective immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
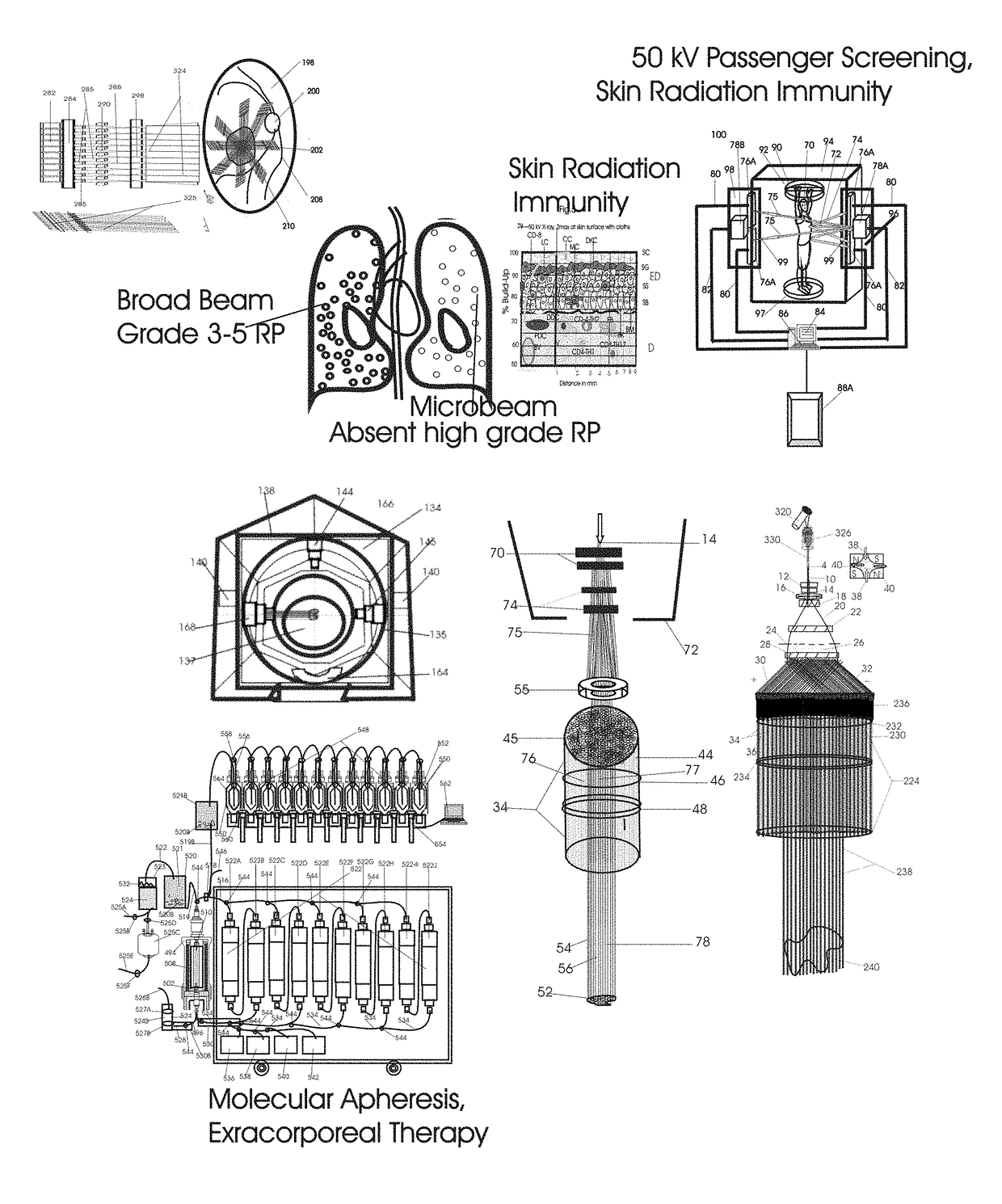
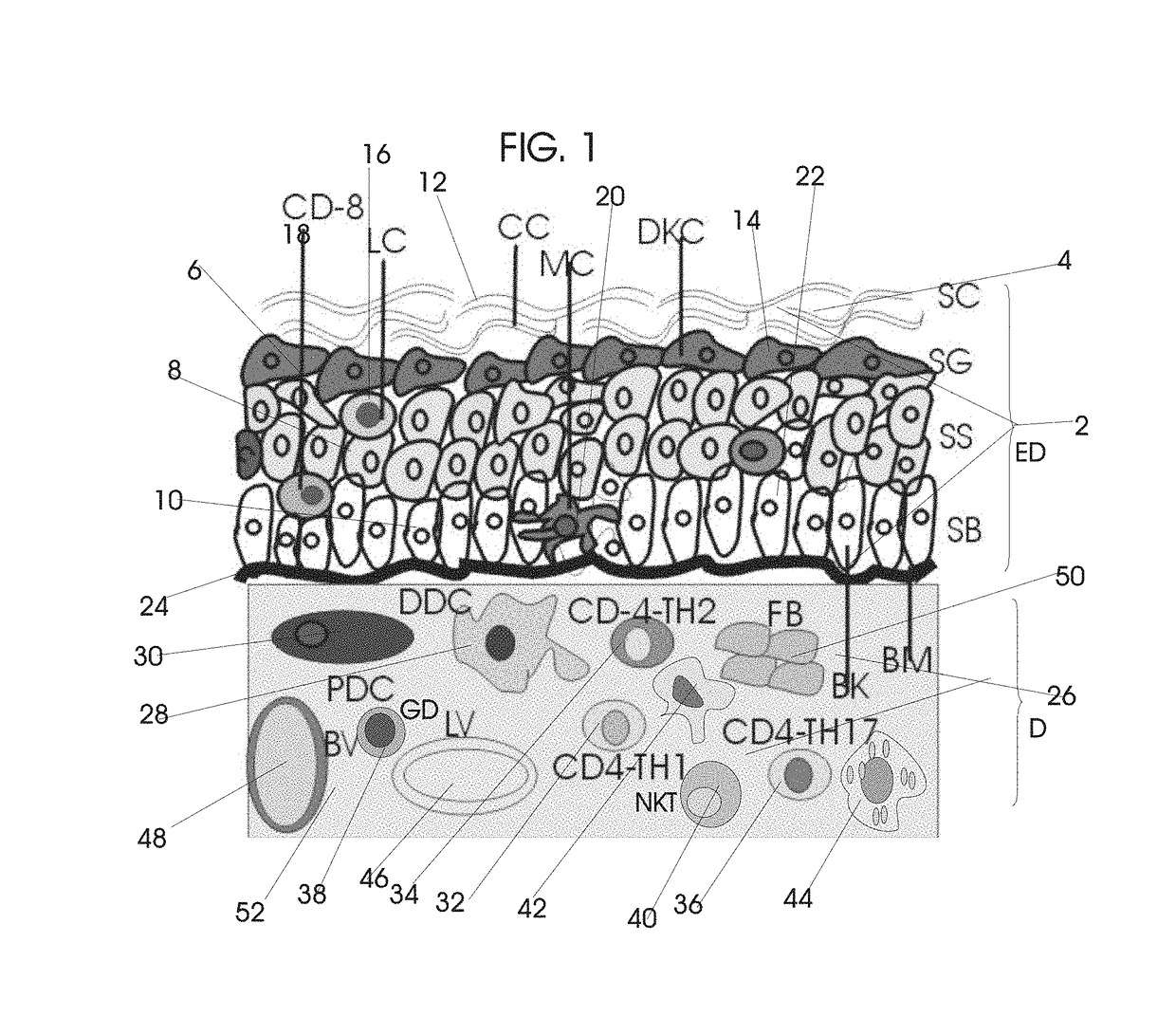
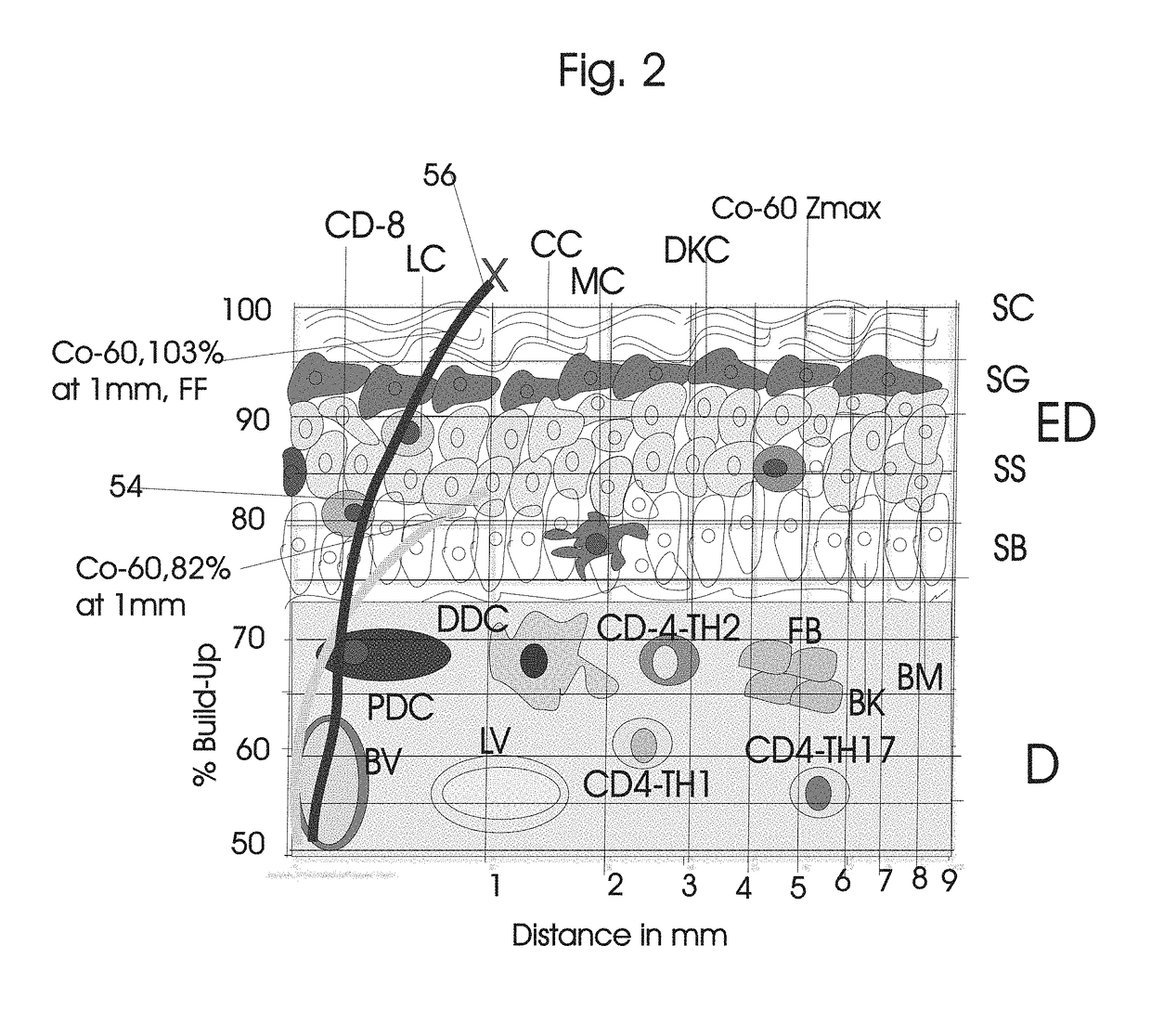
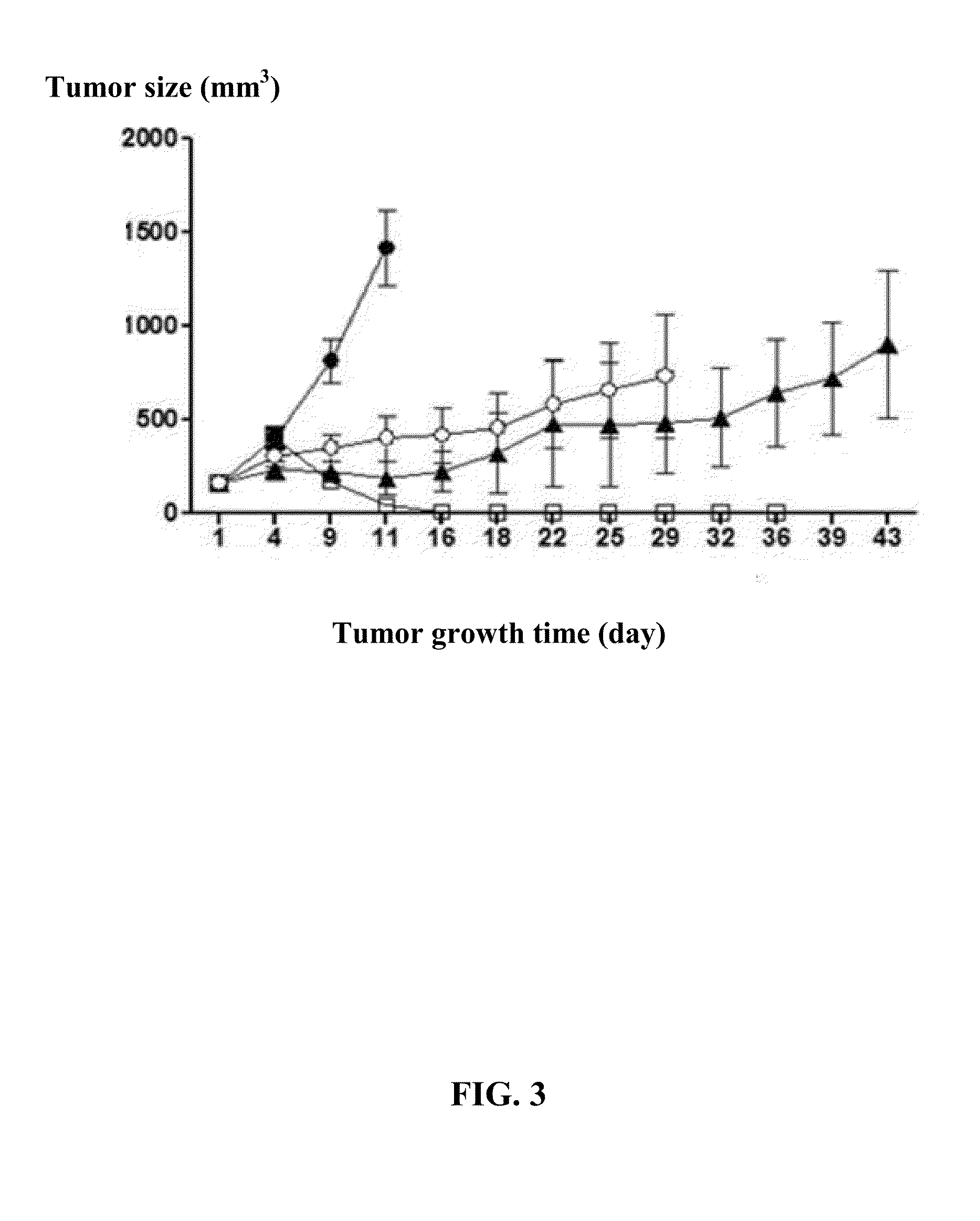
Normal Tissue Toxicity Reducing Microbeam-Broadbeam Radiotherapy, Skin's Radio-Response Immunotherapy and Mutated Molecular Apheresis Combined Cancer Treatments
InactiveUS20180154183A1Improve treatment outcomesIncreased toxicityOther blood circulation devicesHaemofiltrationAbnormal tissue growthGamma ray
Normal tissue complications limit curative broadbeam radiotherapy to tumors including lung cancer. Radiation retinitis causing blindness limits quality of life and long term survival for patients with ocular melanoma. This invention pertains to alternative, normal tissue sparing 100 to 1,000 Gy microbeam radiations with least normal tissue complications and concomitant radio-immunotherapy by innate immune response of epidermis and dermis to low dose radiation with 50 kV X-rays. Total body skin radiation with former airport passenger screening machines with 50 kV X-ray is disclosed. Microbeams are generated without contaminating scatter and neutron radiations from collinear gamma ray and electron beam produced by inverse Compton interaction with high energy laser and electron beam and from proton and carbon ion beams in tissue equivalent cylindrical collimators. Extracorporeal immunotherapy and chemotherapy and apheresis of mutated subcellular particles released into circulation in response to cancer-therapies are by clinical continuous flow ultracentrifugation combined chromatography.
Owner:SAHADEVAN VELAYUDHAN
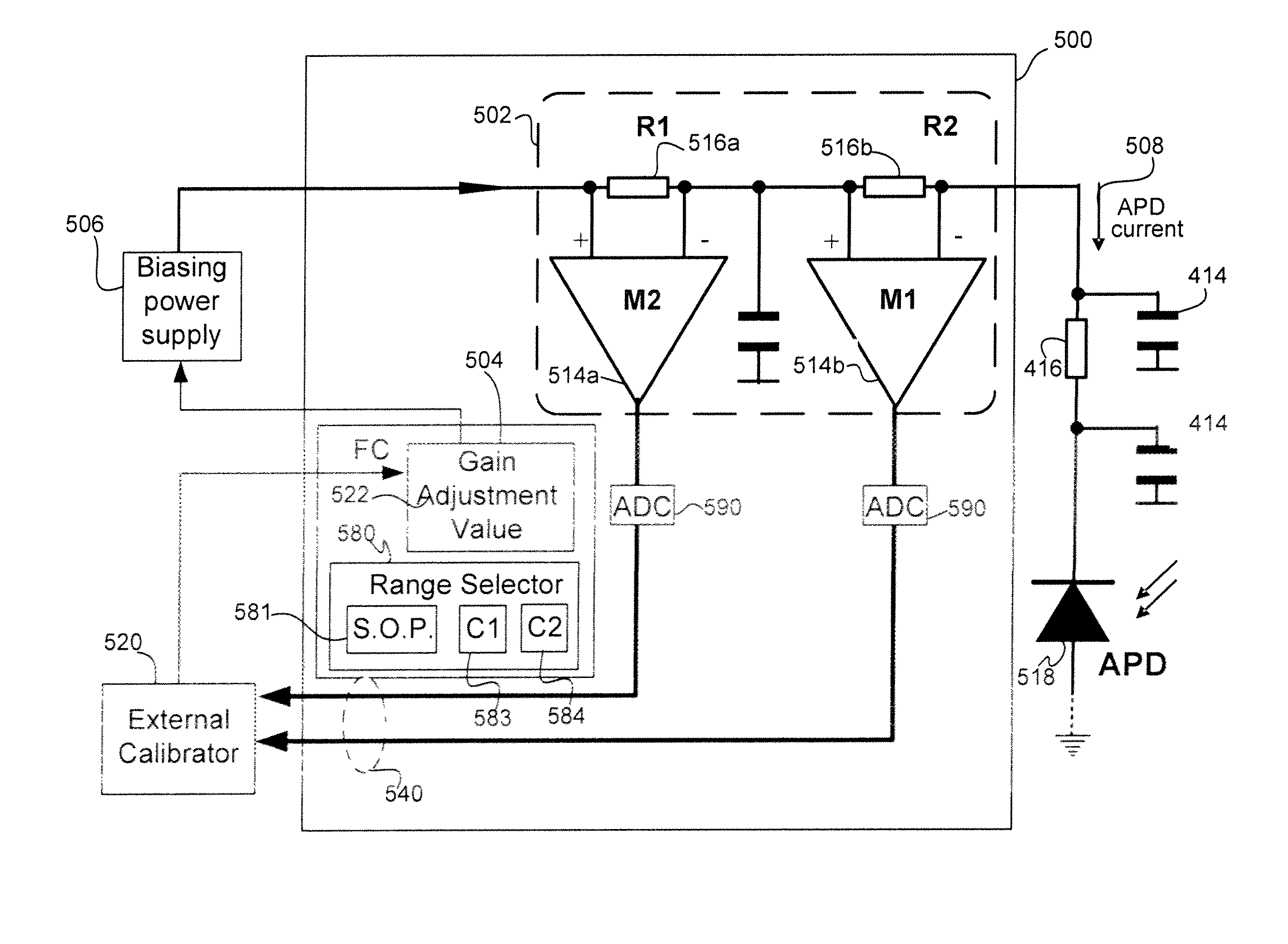
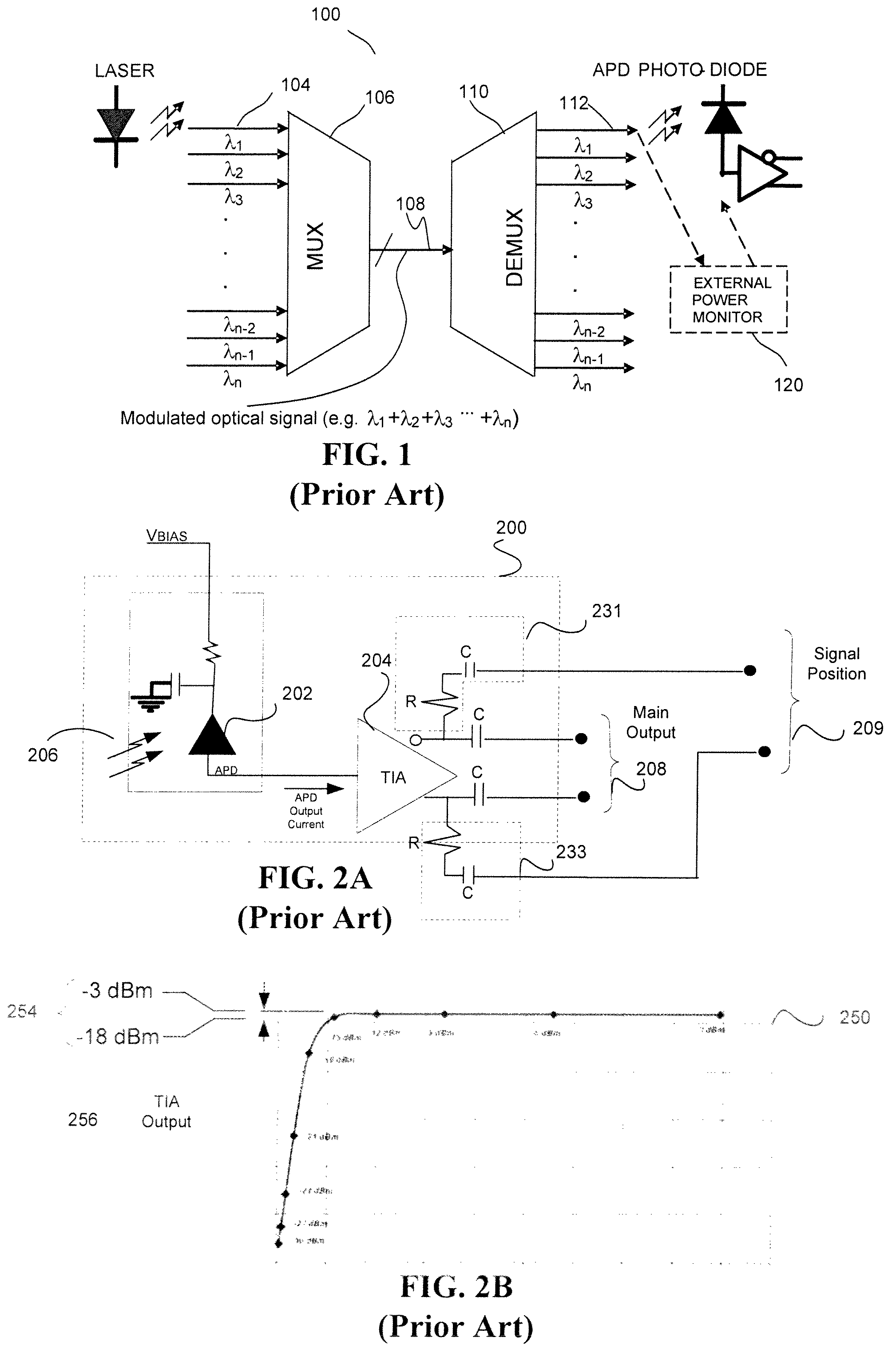
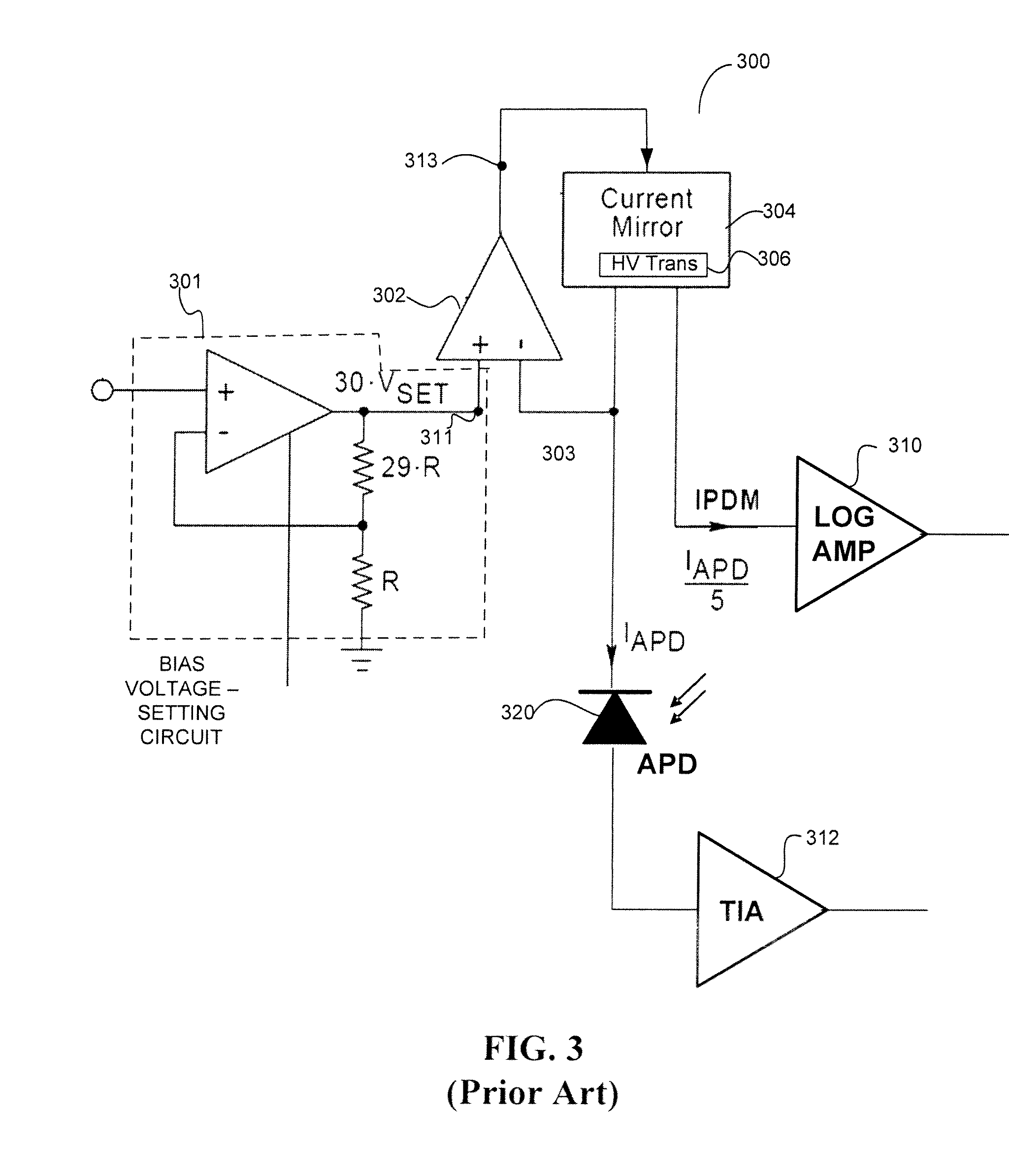
In-situ power monitor providing an extended range for monitoring input optical power incident on avalanche photodiodes
ActiveUS7495203B2Reduce leakage currentEffective immunityGain controlMaterial analysis by optical meansOptical powerPower over
Disclosed are an in-line monitoring apparatus, an optical receiver and a method for monitoring an input power of an optical signal in which one or more power monitoring stages, for example can measure the input power over an extended input power range. In one embodiment, an apparatus includes an avalanche photodiode (“APD”) configured to receive the optical signal and an input configured to bias the APD. It also includes one or more power monitoring stages coupled to the input in parallel with the APD for generating one or more measurement signals in-situ. In one embodiment, a range selector selects which one of the one or more power monitoring stages is to provide a measurement signal indicative of the input optical power. The power monitoring stages can provide for a wide range of linear current measurements as well as a range of measurable currents to monitor low-powered optical signals.
Owner:ALLIED TELESIS
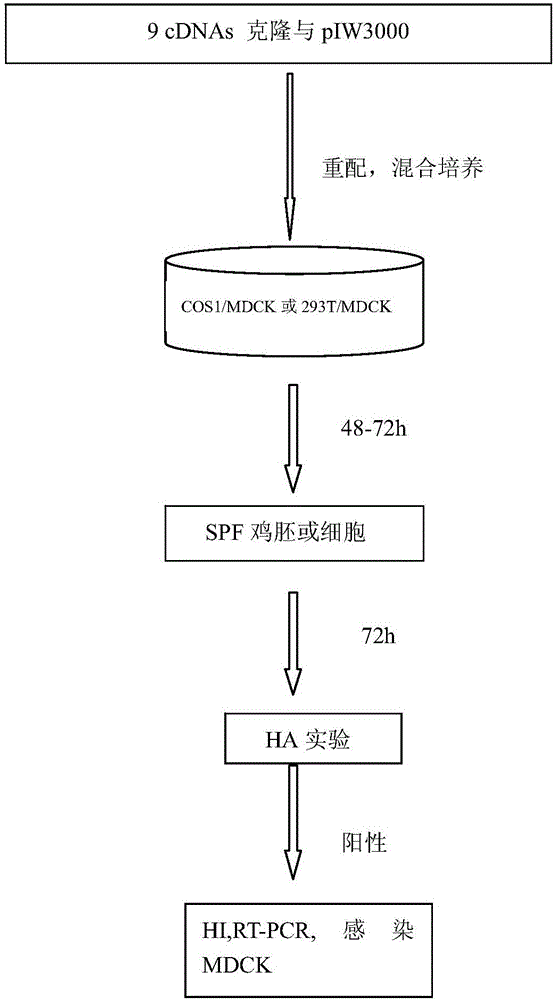
Preparation method and application of recombinant cold-adaptation attenuated influenza vaccine strain
InactiveCN105886529ARapid reorganizationControllable positioningSsRNA viruses negative-senseViral antigen ingredientsHemagglutininImmune effects
The invention discloses a preparation method and an application of a recombinant cold-adaptation attenuated influenza vaccine strain. The method comprises the following steps: importing an influenza virus hemagglutinin HA gene, an influenza virus neuraminidase NA gene as well as seven genes, namely PB2, PB1, PA, NP, M, NS1 and NS2, in an influenza virus to a host cell, and conducting cultivating, so that a recombinant virus is obtained. Tests prove that an attenuated influenza tetravalent vaccine, which is prepared by virtue of a 7+2 plasmid rescuing system, can achieve an excellent immune effect through nasal immunization; the influenza virus, which is recombined by the 7+2 plasmid rescuing system, has a property of being time-efficient; in accordance with the epidemic characteristics of influenza viruses in various years, effective targeted influenza vaccines can be prepared; and the preparation method is rapid, obvious in immunogenicity and immune protection effect, and good in safety; therefore, the problems on safety and stability of attenuated influenza vaccines which are prepared by virtue of a conventional method are solved, and a protection spectrum of the influenza vaccines is more comprehensive.
Owner:CHANGCHUN HAIJIYA BIOTECH CO LTD
Vaccine
InactiveUS20170042997A1Effective immunityMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseVaccination
The present invention relates to a pharmaceutical combination of compositions for use in the treatment or prevention of a disease having cells bearing a target antigen as a vaccine and to a method for vaccination of a mammal, especially of a human for raising a cellular immune response directed against cells of the mammalian recipient, especially human recipient, which cells express a target antigen. The target antigen can e.g. be an autoantigen like a malignant antigen, i.e. a tumour-specific antigen. The pharmaceutical combination of compositions comprises a first composition and a second composition, wherein the second composition is for administration to recipient subsequent to the administration of the first composition, e.g. 2 to 10 days after the first composition. The pharmaceutical combination of compositions has the advantage of raising an effective antigen-specific T-cell response against cells bearing a target antigen that can be a malignant autoantigen, e.g. for raising an antigen-specific T-cell response against cells bearing a tumour-antigen. A further advantage is that the pharmaceutical combination of compositions can raise an antigen-specific T-cell response within a comparatively short time.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER
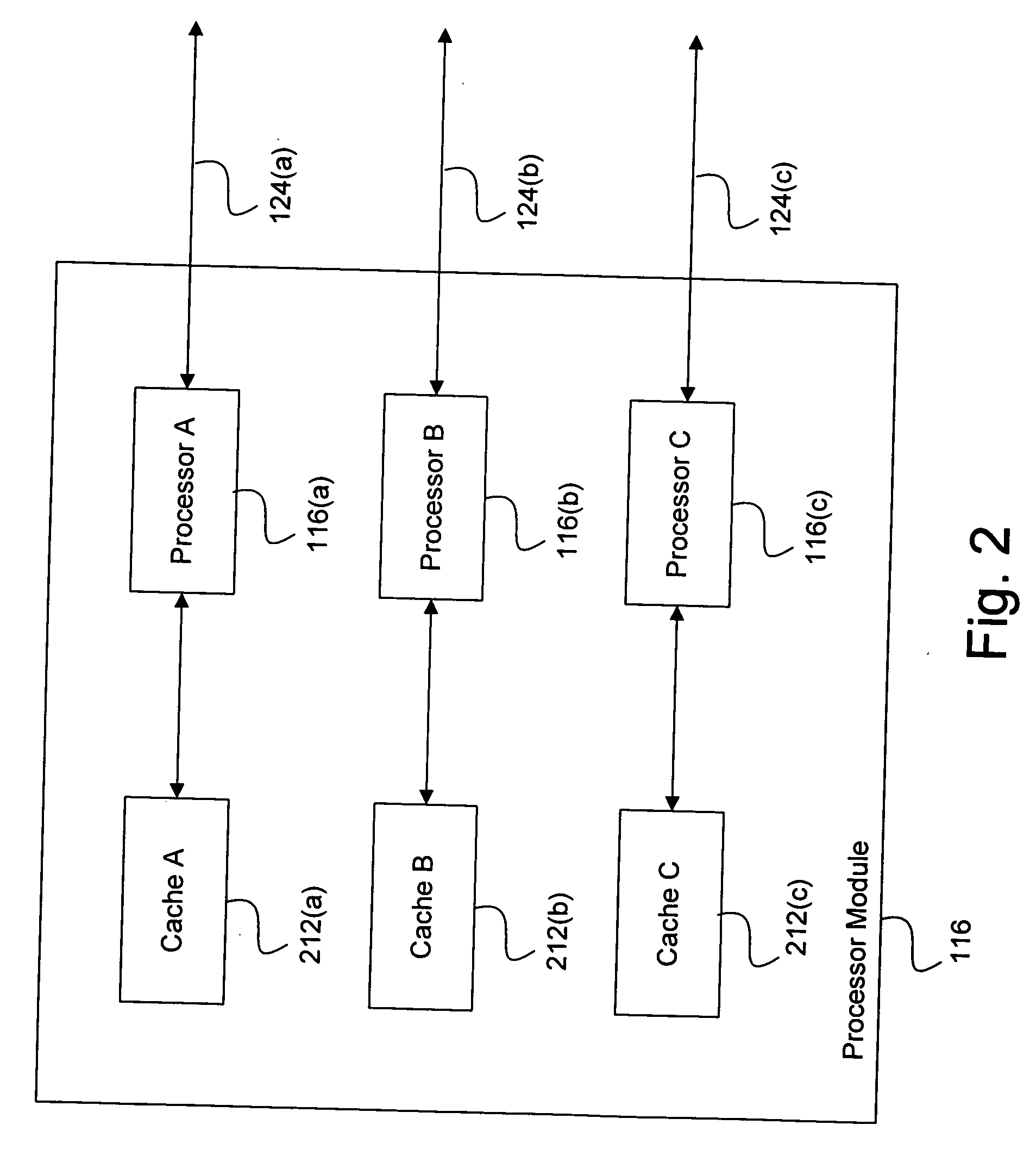
System and method for effectively implementing an immunity mode in an electronic device
InactiveUS20060184824A1Effective immunitySimple methodMemory architecture accessing/allocationError detection/correctionProtection procedureComputer hardware
A system and method for effectively implementing an immunity mode in an electronic device includes a processor module for executing processing tasks for the electronic device. The processor module includes processor information, such as processor states and processor data, for executing the processing tasks. The electronic device also includes a protected memory for storing electronic information in an optimally secure manner. An immunity manager may then perform protection procedures to store at least a portion of the vulnerable processor information into the protected memory in response to an immunity mode trigger event such as the processor module entering an idle state.
Owner:DATA DEVICE CORP
Vaccine composition for mucosal administration
InactiveUS20140234377A1Improve complianceImprove immunityPeptide/protein ingredientsGenetic material ingredientsImmuno modulationPyrimidine Nucleotides
The present invention provides a vaccine composition which comprises a cellular immunity induction promoter universally usable against various antigens in cellular immunity induction by mucosal administration of the antigen and exerts a high cellular immunity inducing effect by mucosal administration. The present invention provides a vaccine composition for mucosal administration to induce cellular immunity, comprising: (i) an antigen; and (ii) one or more cellular immunity induction promoters selected from the group consisting of a TLR ligand, a cyclic dinucleotide, a helper peptide and an immunomodulatory small molecule drug.
Owner:NITTO DENKO CORP
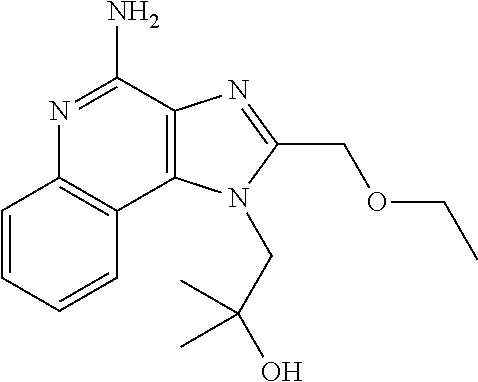
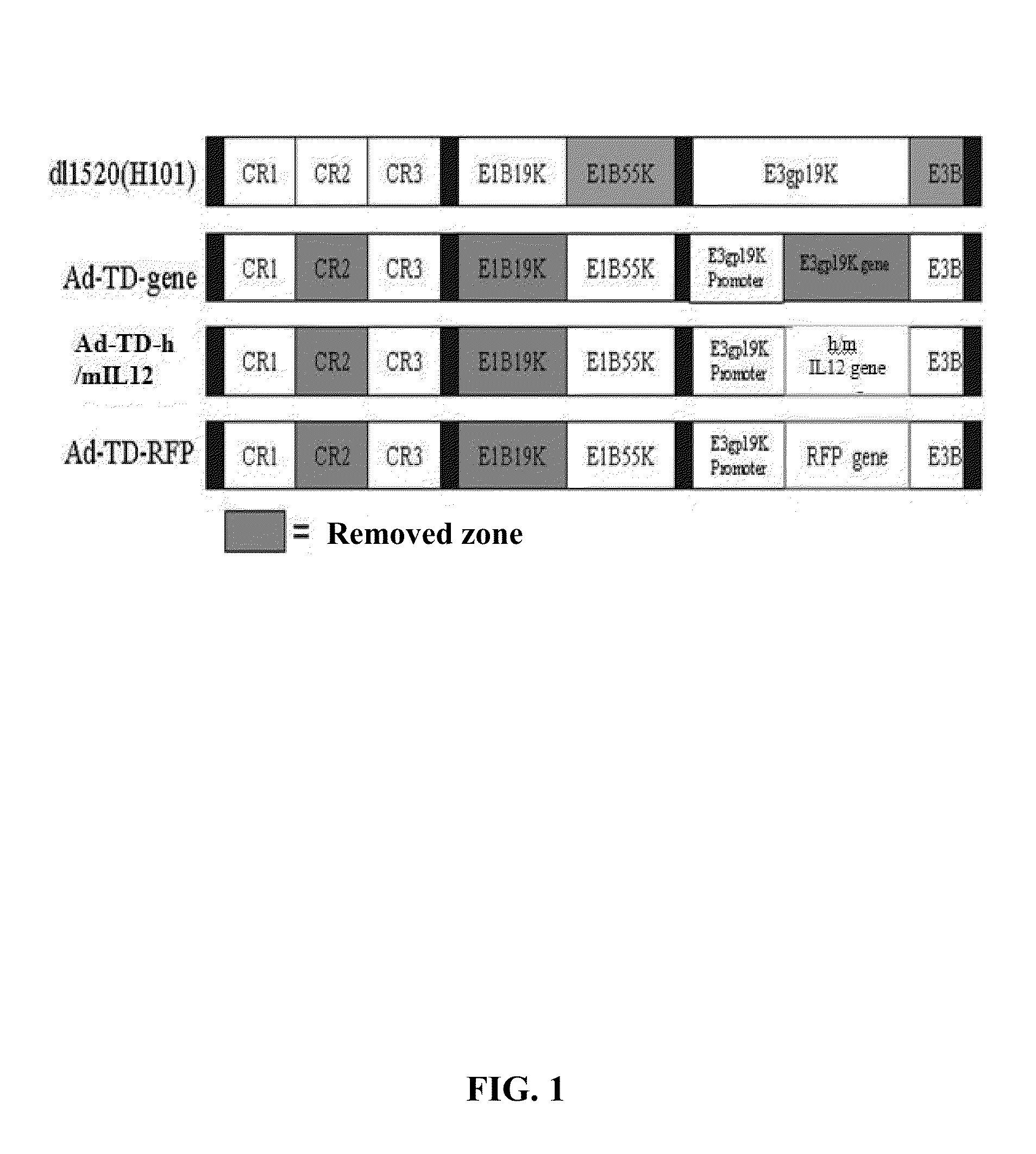
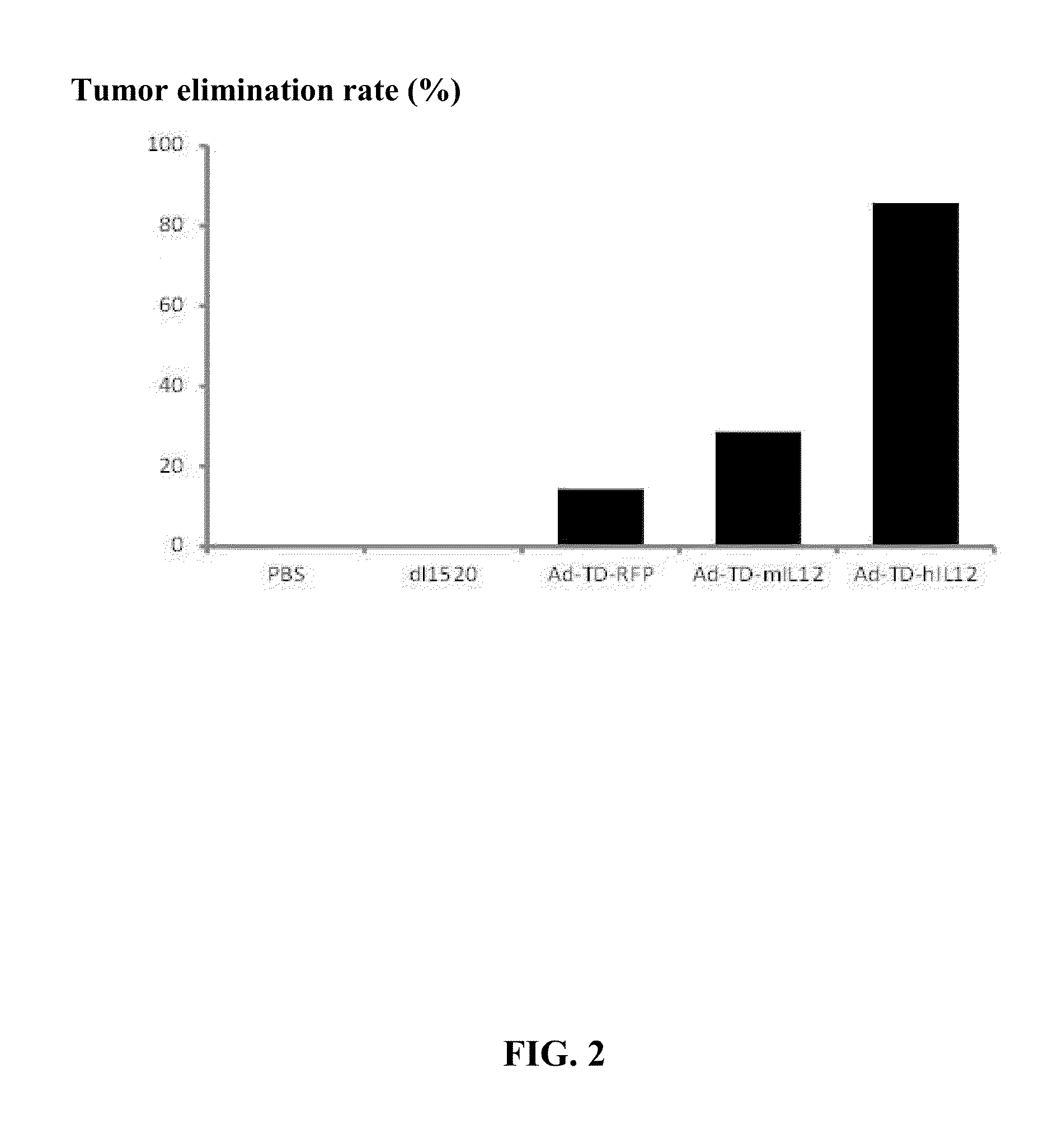
Targeted oncolytic adenovirus for treatment of human tumors, constrcution method and application thereof
InactiveUS20130345295A1Safe and highly efficientEffective immunityOrganic active ingredientsPeptide/protein ingredientsGenetic engineeringSide effect
An oncolytic adenovirus vector and its potential application in cancer treatment and vaccination. The inventive vector (named Ad-TD-hIL12) is derived from the human adenovirus group C type 5, more particularly including deletion of three adenovirus genes E1A-CR2, E1B19K and E3gp-19K, and a fused cDNA sequence of p35 and p40 subunit of human IL12 placed under the control of the E3gp-19K promoter. The invention also includes a method to construct the triple gene-deleted vector (Ad-TD). The Ad-TD-hIL12 and Ad-TD-shIL12 (with a short p40 sequence of human IL 12) vectors can be used as targeted, genetically engineered agents for treatment of various solid tumors, via not only intratumoral injection, and also in intraperitoneal injection, without causing significant side effects, showing a superior antitumor efficacy and safety.
Owner:BEIJING BIO TARGETING THERAPEUTICS TECH
Continuous self-test of a gyroscope
ActiveUS20200400433A1Effective immunityFast error detection timeSpeed measurement using gyroscopic effectsGyroscopes/turn-sensitive devicesGyroscopeSoftware engineering
A microelectromechanical gyroscope includes a drive loop having a drive element and a drive loop circuitry. The drive loop circuitry includes a clock generating circuitry for generating from the quadrature-phase detection signal a test clock signal, an angular rate phase demodulation signal and a quadrature phase demodulation signal. A sense loop includes a sense element and sense loop circuitry for detecting angular rate and producing a force-feedback signal. A test signal generator receives a quadrature-phase detection signal to be used as a quadrature-phase carrier signal and the test clock signal A summing element sums a test signal with the force-feedback signal to form a sense feedback signal. A rate phase demodulator produces a rate signal by demodulating a sense signal received from the sense loop with the angular rate phase demodulation signal, and a quadrature-phase demodulator produces a quadrature-phase output signal.
Owner:MURATA MFG CO LTD
Recombination attenuated salmonella typhimurium carrier vaccine of expression IBDV (Infectious Bursal Disease Virus) immunogenic gene and preparation method thereof
InactiveCN101920011AImproving immunogenicityImprove protectionBacteriaViral antigen ingredientsVector vaccineInfectious bursitis
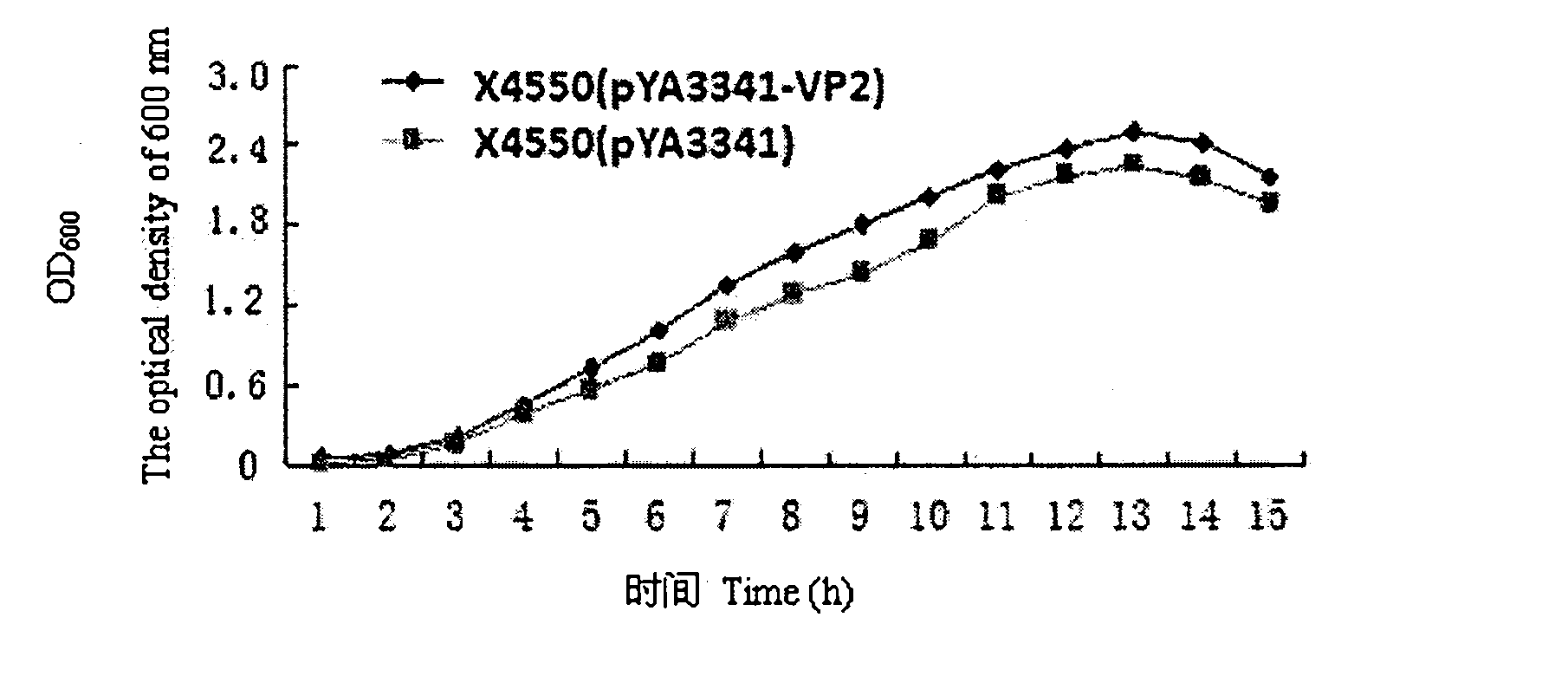
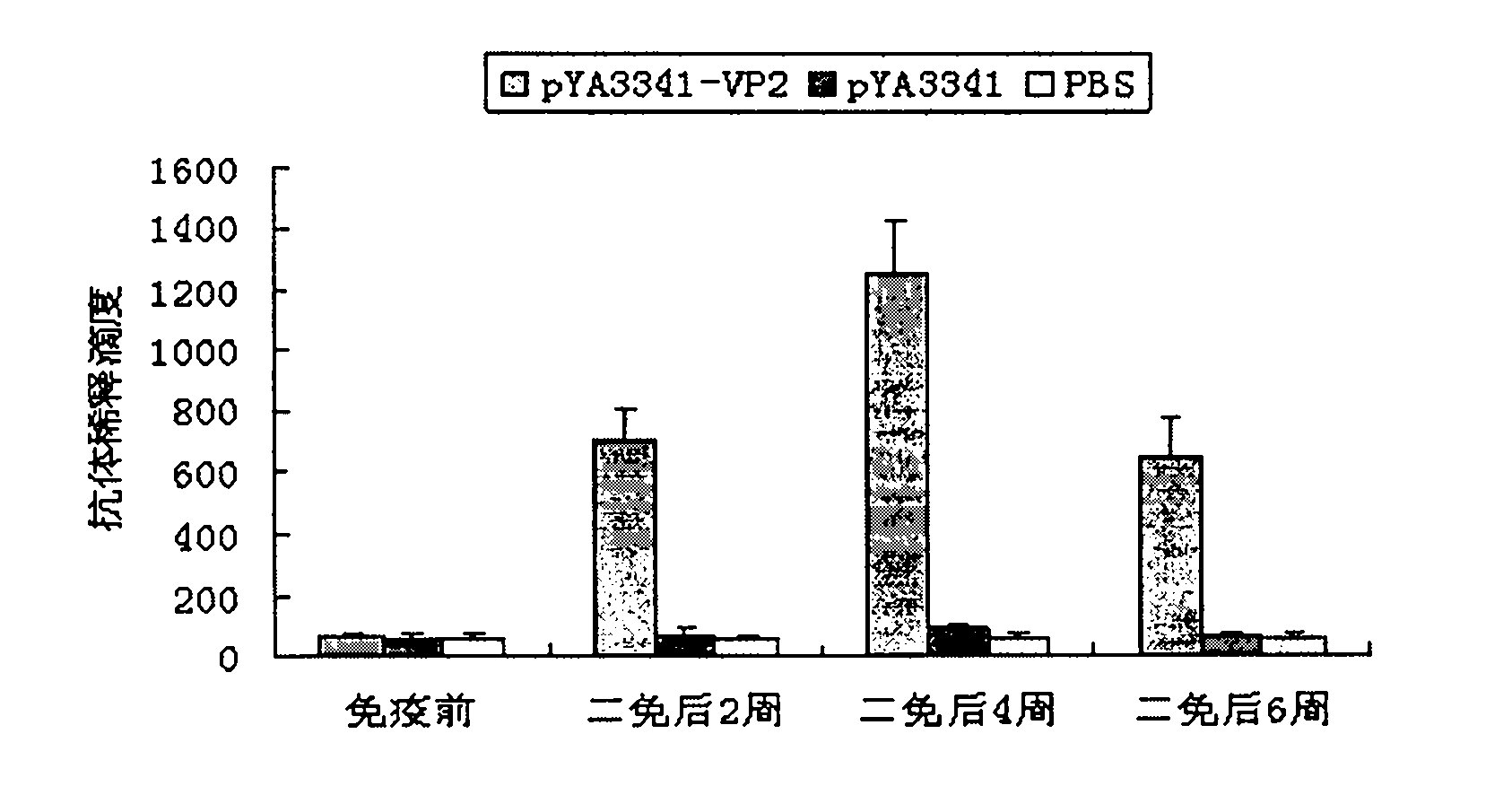
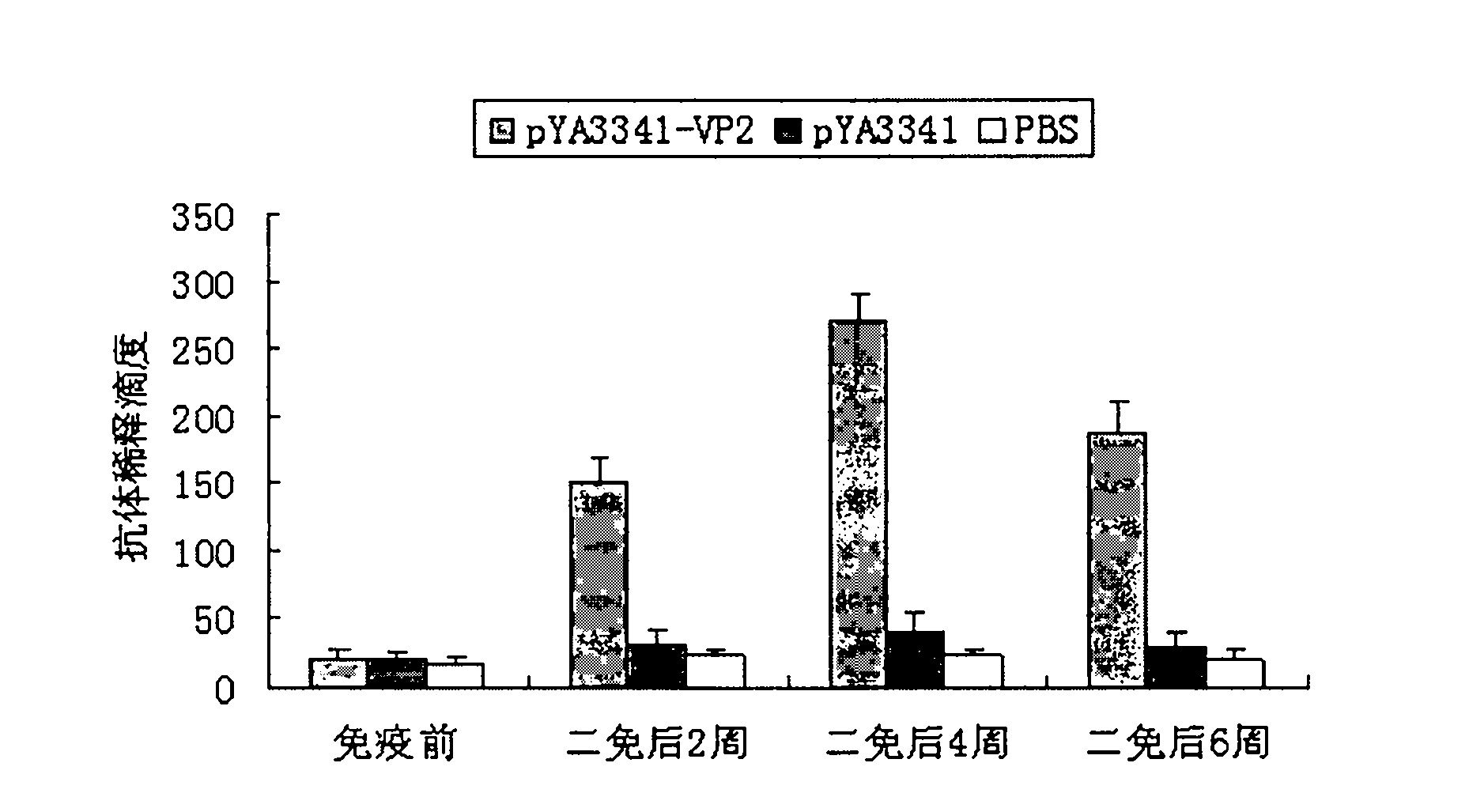
The invention discloses a recombination attenuated salmonella typhimurium carrier vaccine of expression IBDV (Infectious Bursal Disease Virus) immunogenic gene, a preparation method and application thereof, in particular relates to oral attenuated salmonella typhimurium. The recombination attenuated salmonella typhimurium comprises a sequence disclosed by SEQ ID NO.1, and the recombination virus can express IBDV capsid protein VP2. The preparation method comprises the following steps of: inoculating the attenuated salmonella typhimurium in an LB substrate for culturing 18 hours; regulating bacterial concentration as 1010CFU / ml; and preparing the safe and effective oral attenuated salmonella typhimurium carrier vaccine for preventing IBD (Infectious Bursal Diseases).
Owner:NORTHWEST A & F UNIV
Attenuated ehrlichiosis vaccine
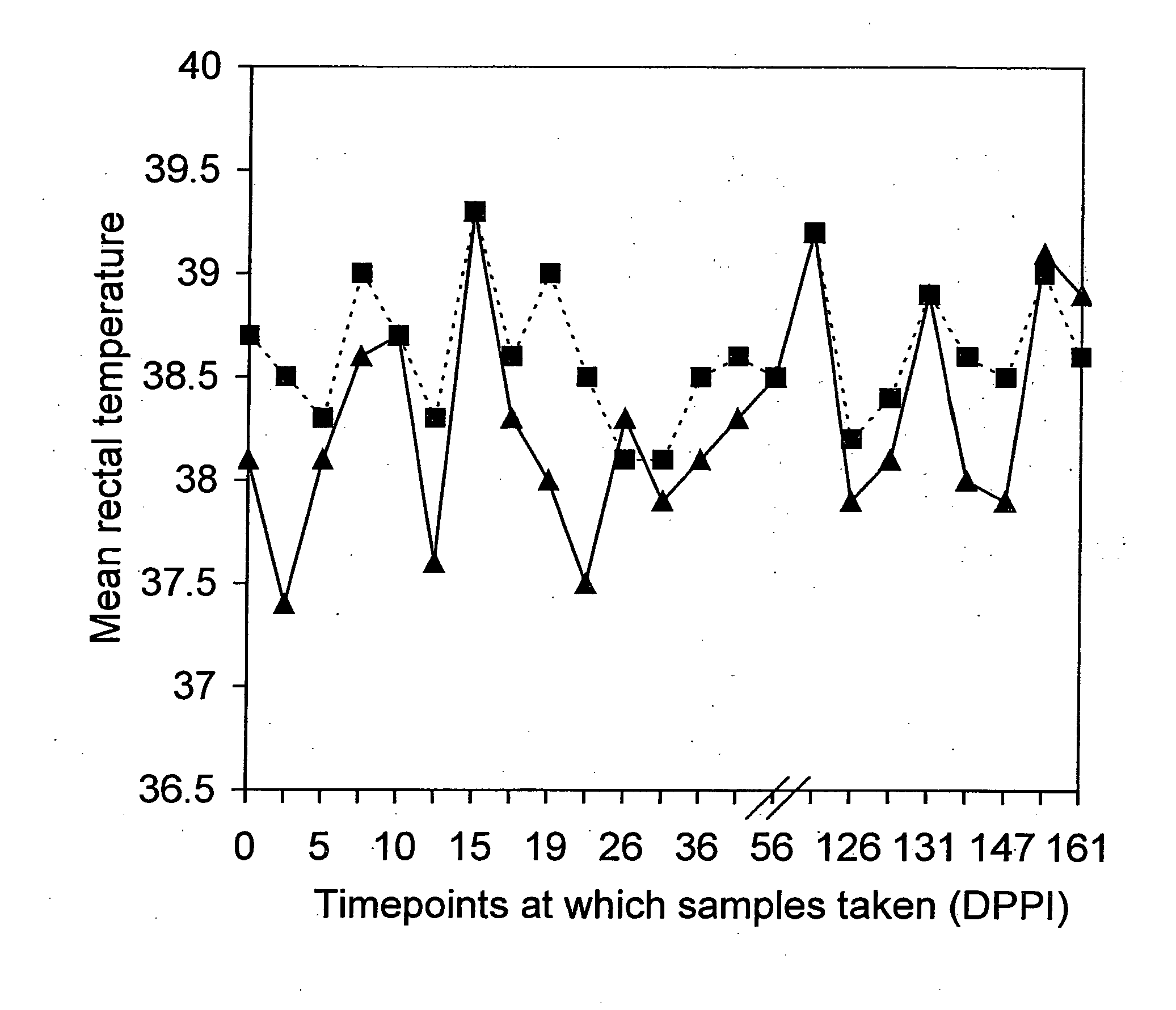
ActiveUS20100239613A1Avoid problemsEffective immunityAntibacterial agentsBacterial antigen ingredientsAttenuated strainBiology
The present invention relates to an attenuated strain of Ehrlichia canis and a vaccine comprising said attenuated strain for protection of mammals against ehrlichiosis. The invention further relates to methods of preventing ehrlichiosis and of attenuating the pathogenicity Ehrlichia canis.
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
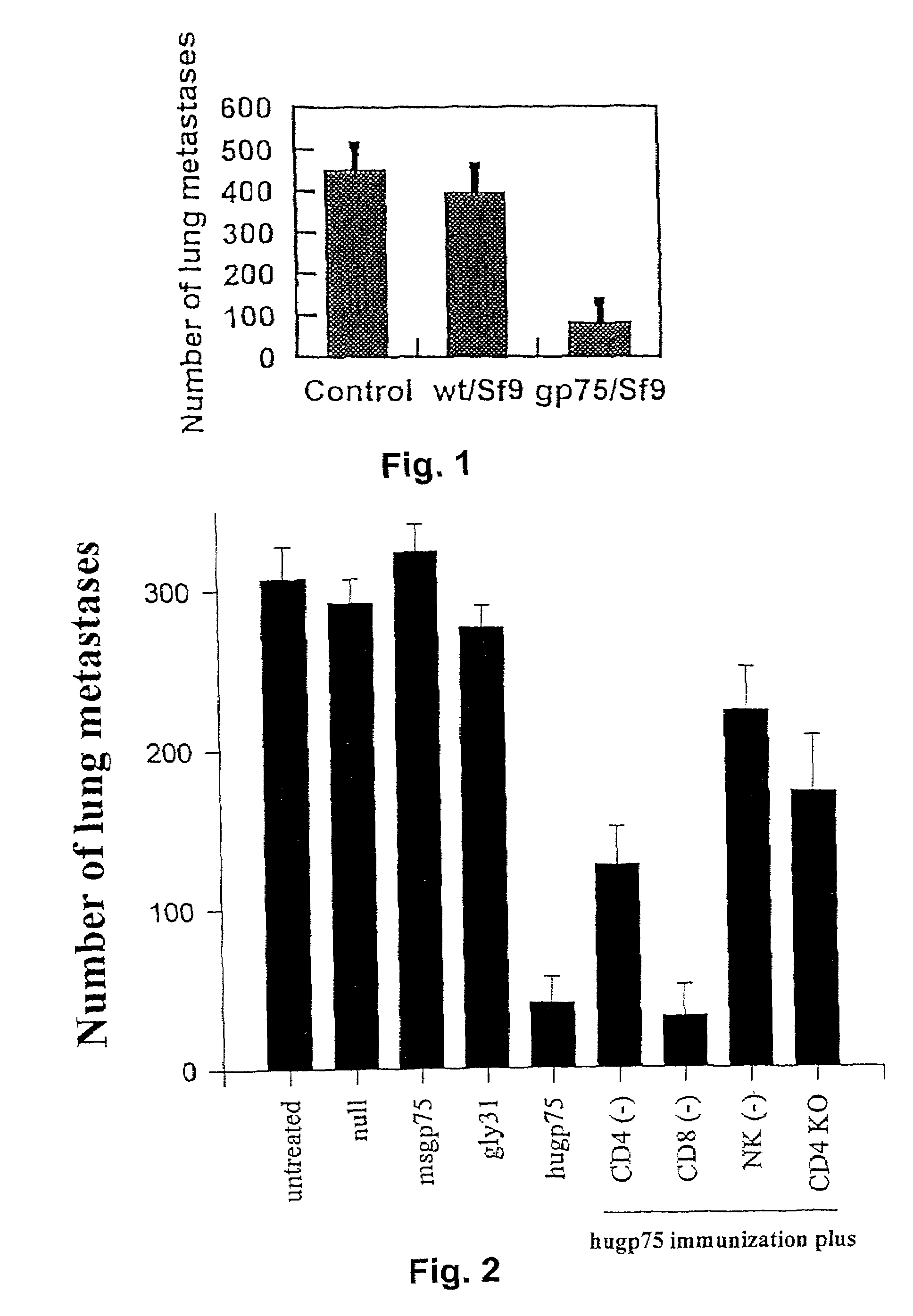
Compositions for treatment of melanoma and method of using same
InactiveUS7556805B2Overcome toleranceEffective immunityBiocideBacteriaDifferentiation AntigensMelanoma
Melanoma can be treated in a mammalian subject by administering to the subject an immunologically-effective amount of a xenogeneic melanoma-associated differentiation antigen. For example, genetic immunization with a plasmid containing a sequence encoding human gp75 has been shown to be effective in treatment of dogs with melanoma.
Owner:ANIMAL MEDICAL CENT THE
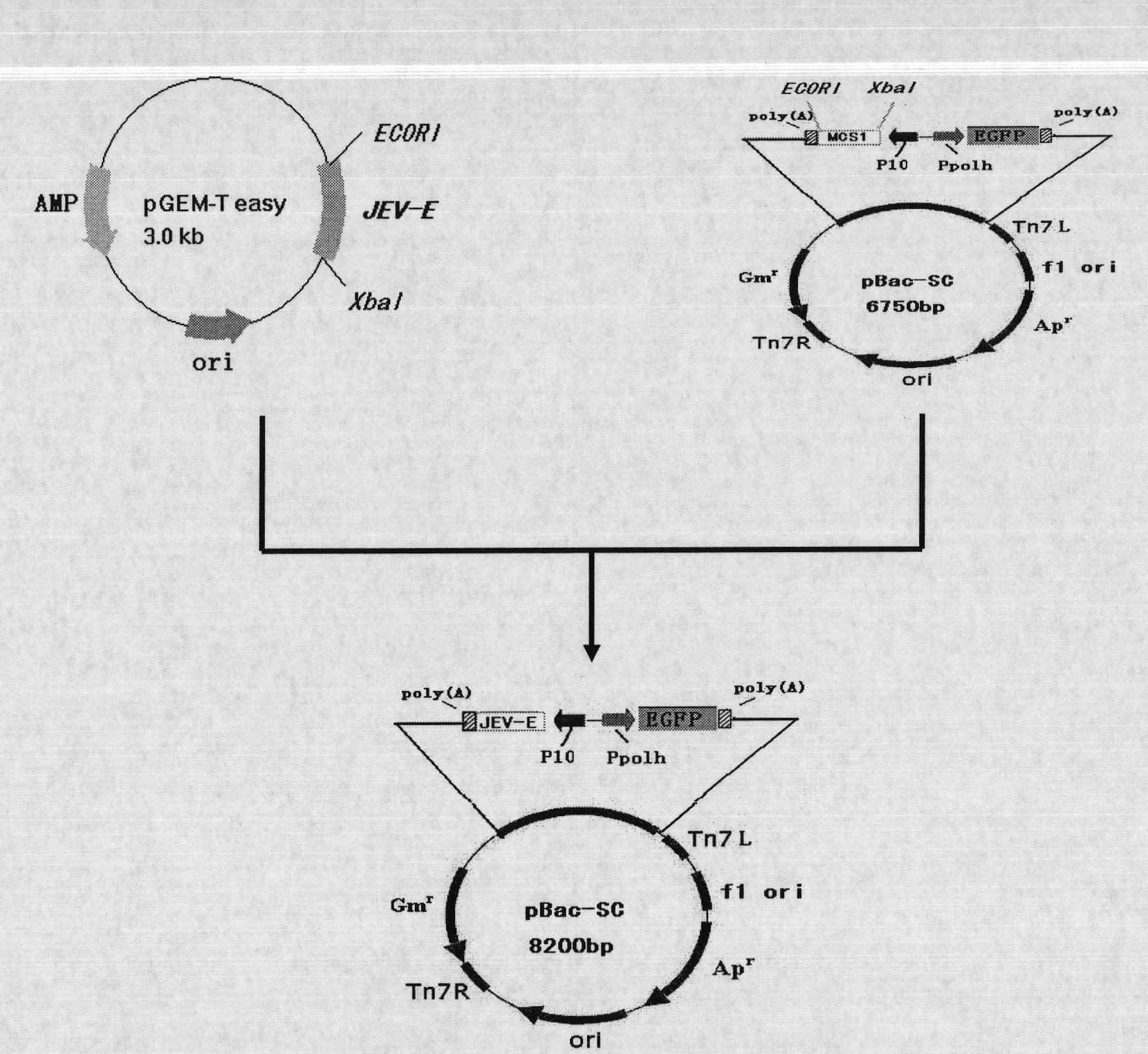
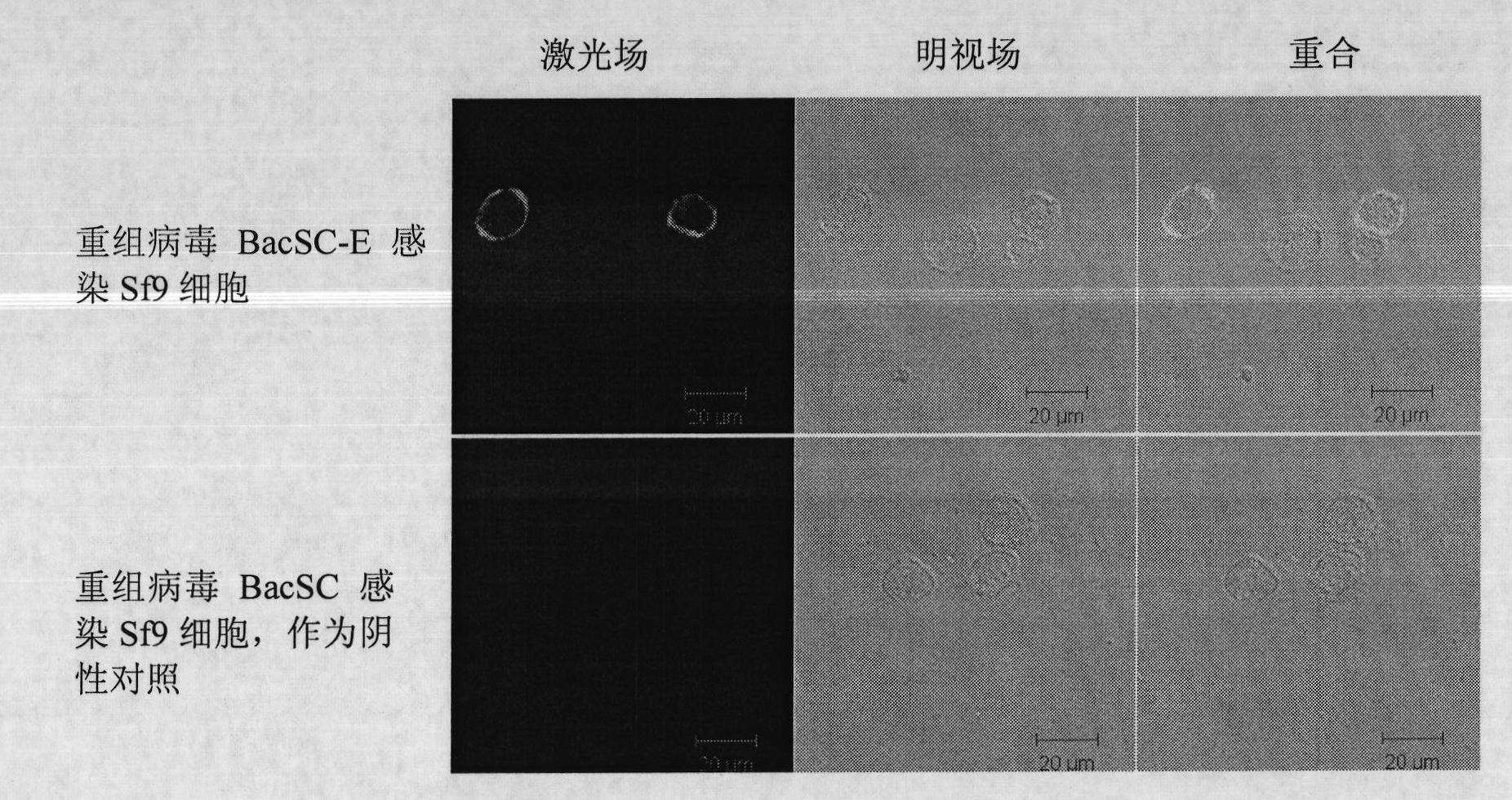
Recombinant baculovirus expressing Japanese Encephalitis Virus (JEV) immunogen gene and preparation method and application thereof
InactiveCN101792741AAvoid the tedious process of separation and purificationEasy to measureNervous disorderViral antigen ingredientsFreeze thawingStructural protein
The invention discloses a recombinant baculovirus expressing Japanese Encephalitis Virus (JEV) immunogen genes and a preparation method and application thereof, particularly relates to a recombinant baculovirus comprising a sequence shown as SEQ ID NO.1 and displaying structural proteins E of the JEV on the surface. The preparation method comprises steps of utilizing the recombinant baculovirus to inoculate Sf9 insect cells, culturing for 48-72h, collecting infection cells, carrying out freeze thawing at 40DEG C below zero / 37DEG C, centrifuging, taking supernate to test virus PFU and adjusting the titer of the virus to 109PFU / ml to obtain a safe and effective gene engineered subunit vaccine for preventing swine JEV.
Owner:NORTHWEST A & F UNIV
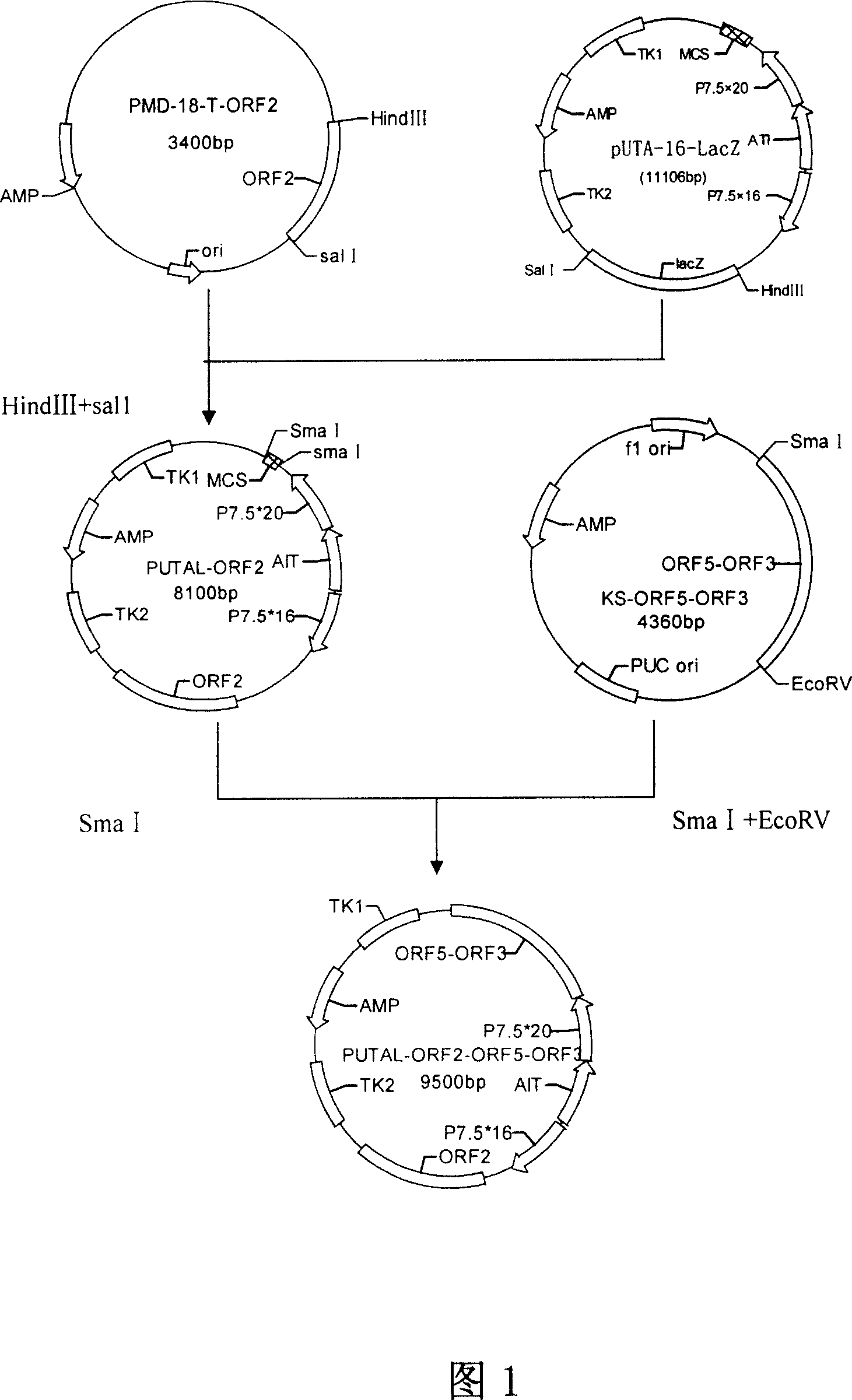
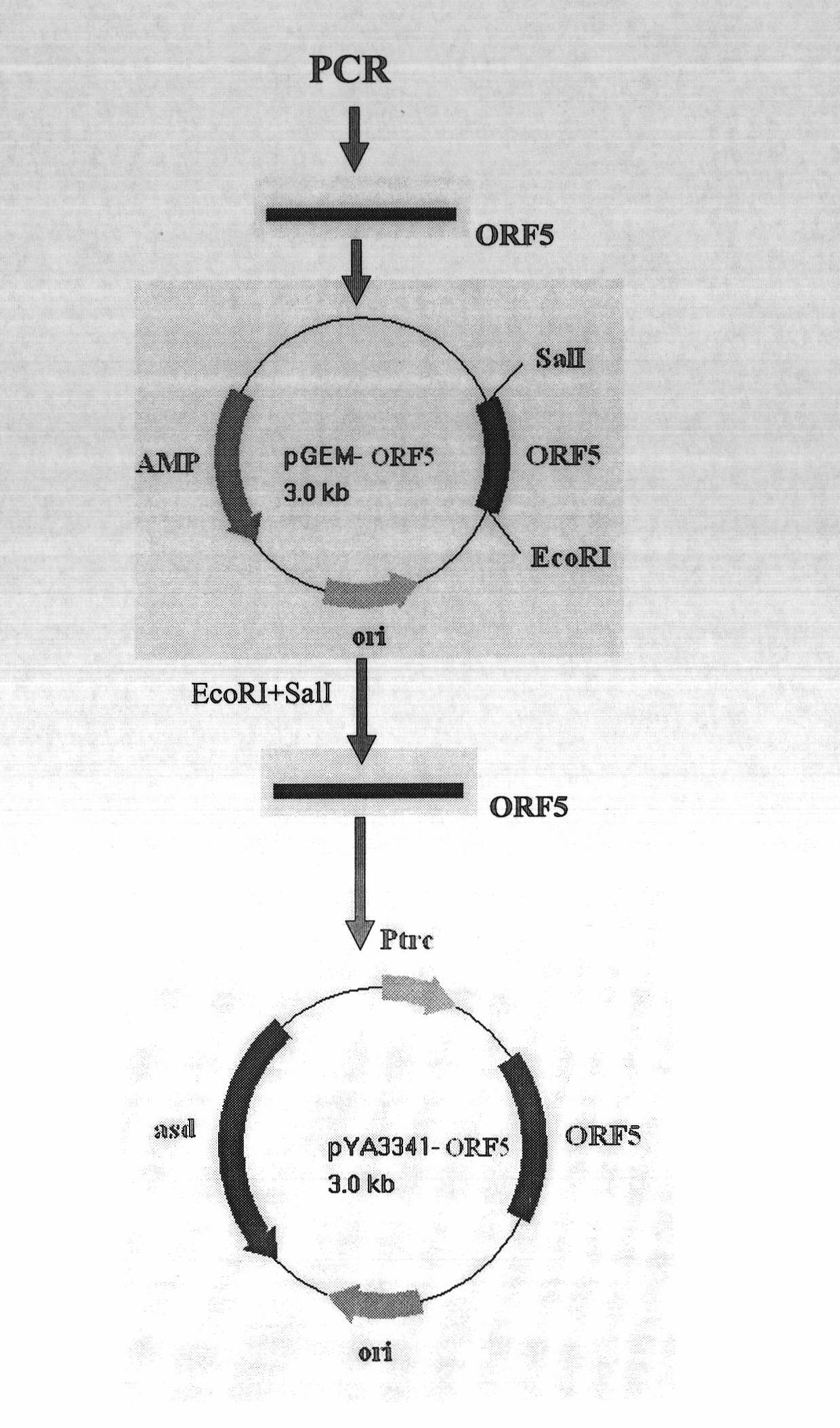
PRRSV and PCV-2bivalent recombinant fowl pox virus disease live vector vaccines
InactiveCN101112621AEffective immunityNo pathologyGenetic material ingredientsRespiratory disorderDiseaseFowl
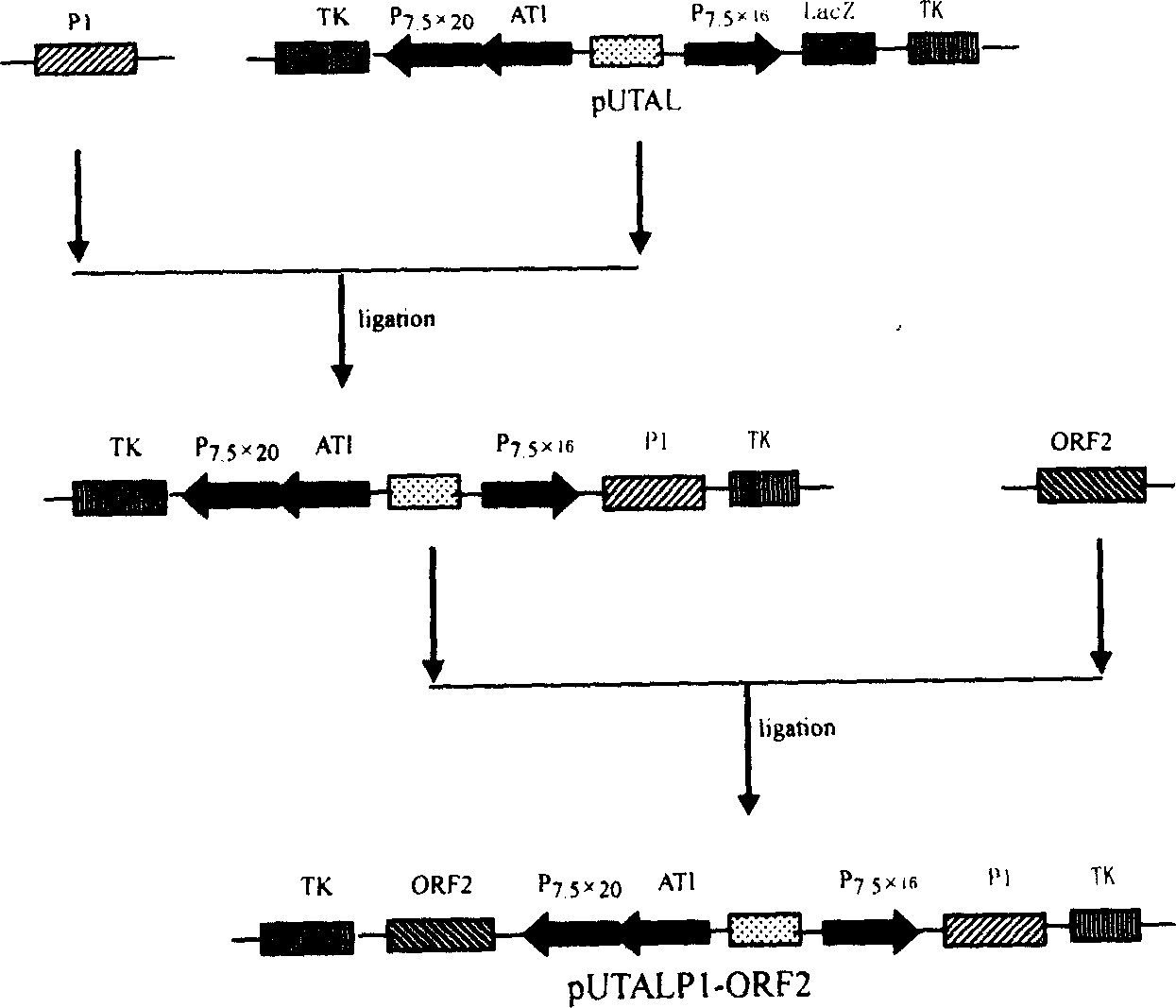
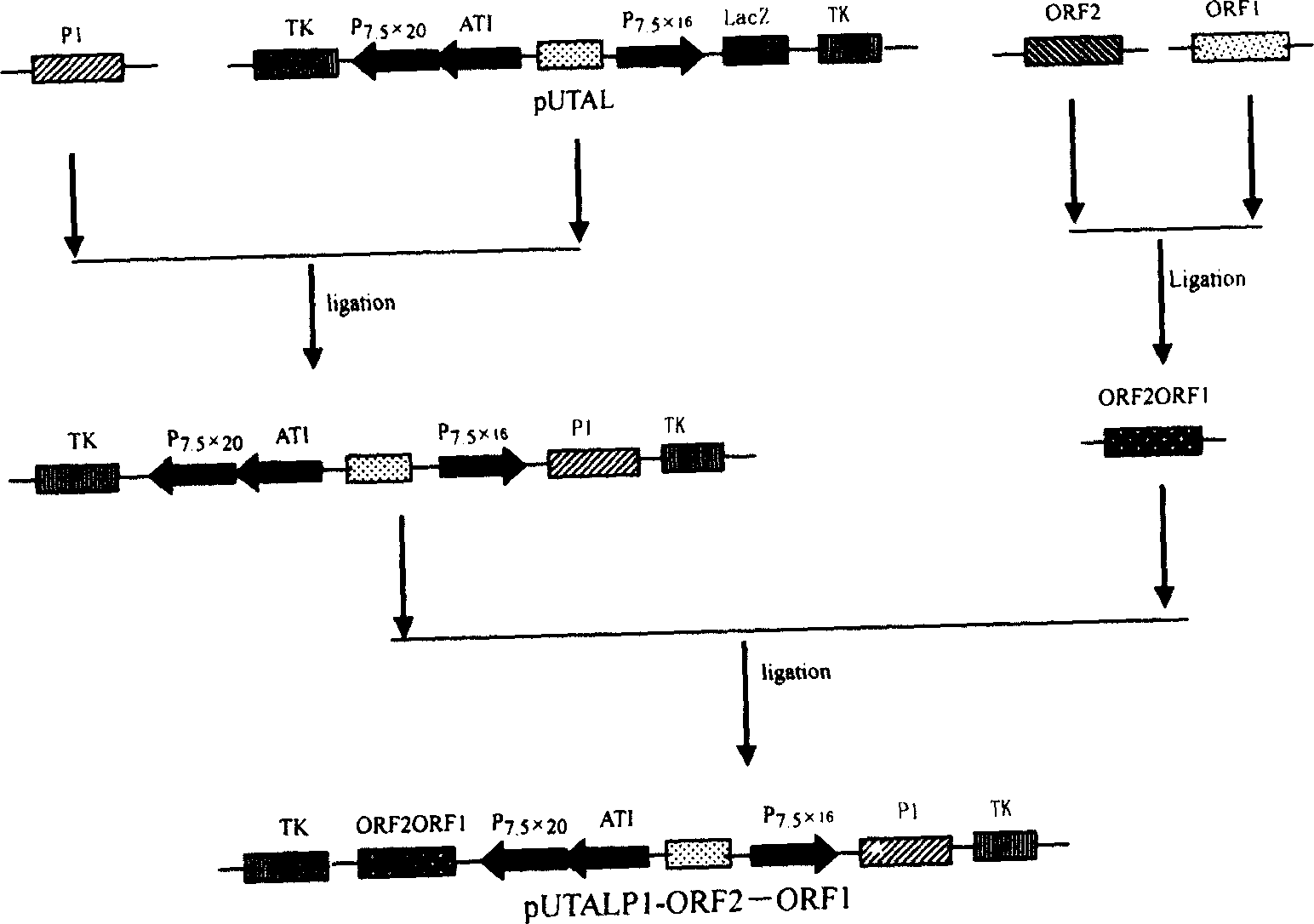
The present invention relates to a PRRSV and PCV-2 bivalent recombinant fowlpox live vector vaccine, which pertains to the field of biotechnology. The present invention aims at providing the PRRSV and PCV-2 bivalent recombinant fowlpox live vector vaccine which has ORF5, ORF3 genes to express the structural glycoprotein of the PRRSV and ORF2 gene to express PCV-2 nucleocapsid protein and can be used as the live vector vaccine for the prevention of PRRS and PCV-2 infection in our country. The present invention constructs the pMD18T-ORF2, pMD18T-ORF5 and pMD18T-ORF3 plasmids, the plasmids are inserted to the downstream of the compound promoter ATI-P7.5 multiplied by 20 of the fowlpox virus expression vector pUTAL, at the same time, the ORF2 gene of the PCV2 are inserted to the downstream of the linking promoter P7.5 multiplied by 16, and the present invention further constructs the recombinant fowlpox virus gene transfer plasmid pUTAL-ORF2-ORF5-ORF3 which contains the ORF3-ORF3 gene of the PRRSV and the ORF2 gene of the PCV2. The present invention can be used as the live vector vaccine for the prevention of PRRS-PCV2 in our country.
Owner:金宁一
Method for preparing ginseng polysaccharide for immune stimulation and ginseng polysaccharide for immune stimulation prepared therefrom
ActiveUS20170189464A1Effective immunityIncrease incomeOrganic active ingredientsImmunological disordersAqueous alcoholFiltration
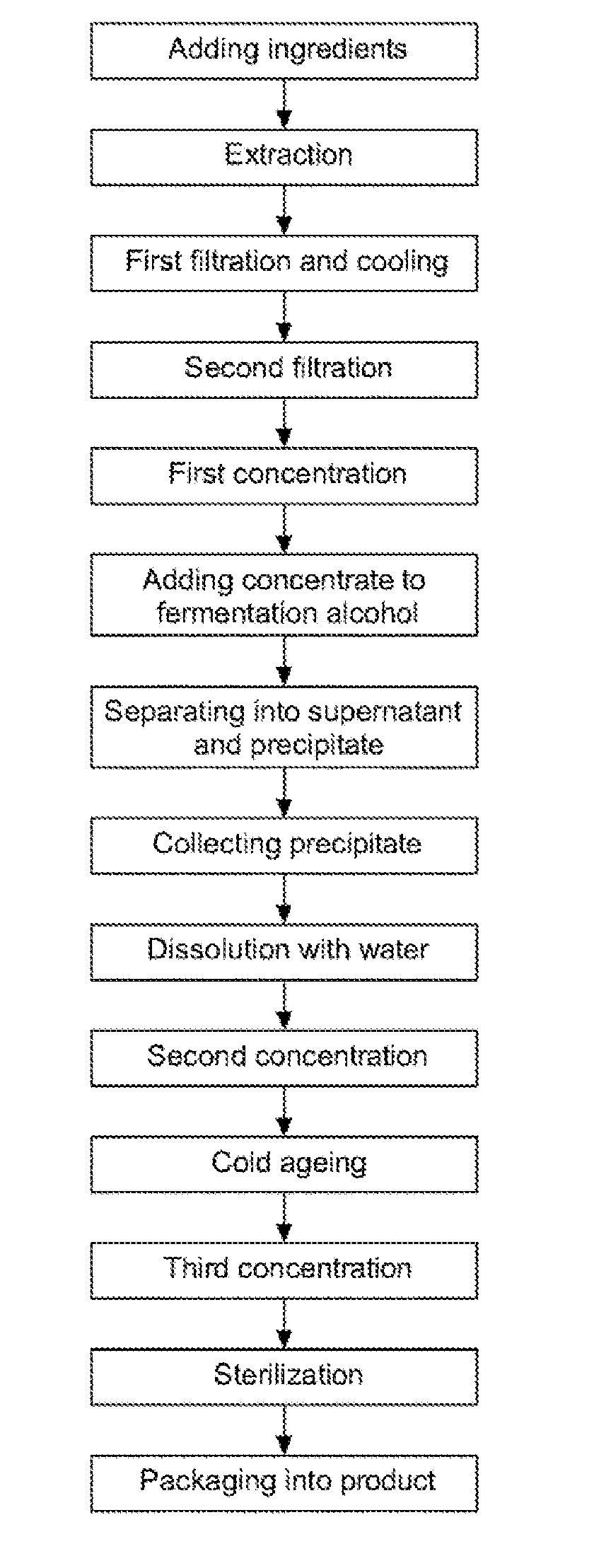
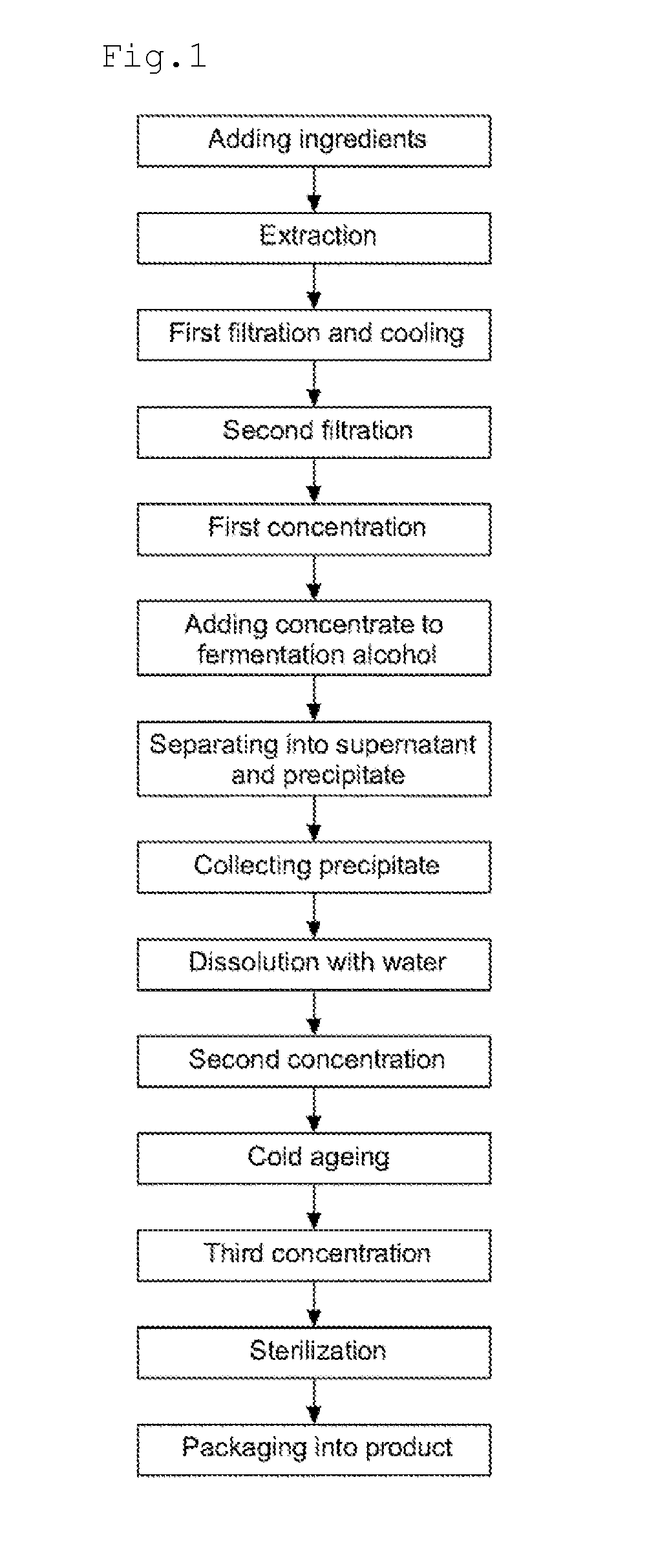
Disclosed are a method for preparing a ginseng polysaccharide for immune stimulation and a ginseng polysaccharide for immune stimulation prepared from the preparation method, the method comprising the steps of (1) adding ingredients; (2) performing an extraction; (3) performing a first filtration and cooling; (4) performing a second filtration; (5) performing a first concentration; (6) adding a concentrate to a fermentation alcohol; (7) separating into a supernatant and a precipitate; (8) collecting the precipitate; (9) dissolving in water; (10) performing a second concentration; (11) cold ageing; (12) performing a third concentration; (13) sterilizing; and (14) packaging into a product of the ginseng polysaccharide for immune stimulation in the form of a concentrate.
Owner:HEALTH BIO MAD
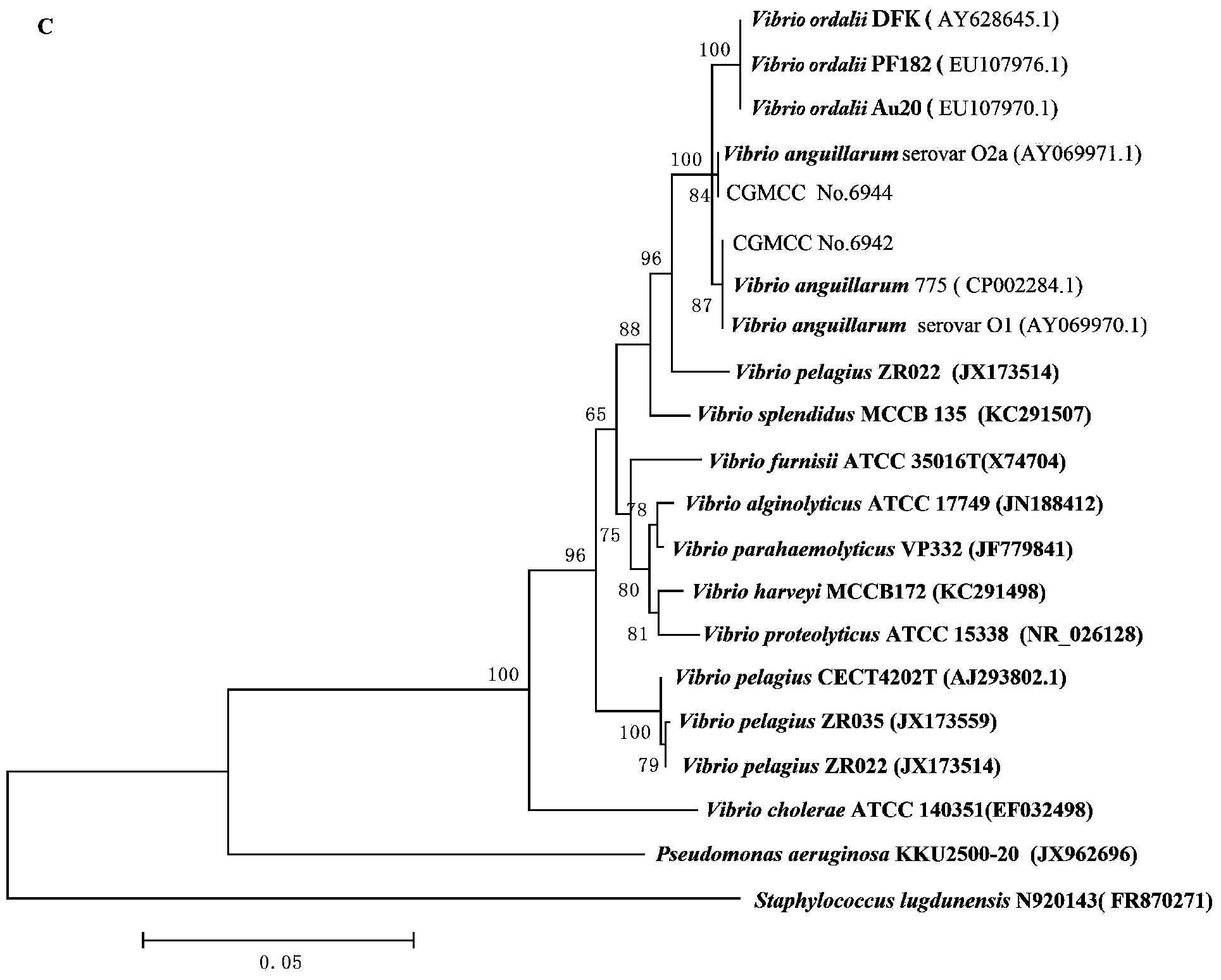
A vibrio anguillarum divalent vaccine, a preparing method thereof and a using method of the vaccine
ActiveCN104138597AExpand the scope of protectionGood securityAntibacterial agentsBacterial antigen ingredientsSerotypeSpecific immunity
The invention relates to aquaculture animal disease control technologies, and particularly relates to a vibrio anguillarum divalent vaccine, a preparing method thereof and a using method of the vaccine. The vaccine is a bacterial suspension mixture of a vibrio anguillarum 01 serotype and a 02 serotype. The vibrio anguillarum 01 serotype and the 02 serotype are deposited in the general microbiology center of the China Committee for Culture Collection of Microorganisms on December 7, 2012 and respectively have an accession number namely CGMCC No.6942 and CGMCC No.6944. The divalent inactivated vaccine can protect susceptible fishes from being infected by pathogenic vibrio anguillarum. The divalent vaccine broadens the vaccine protection range, effectively irritates cultured fishes to generate specific immune response, and effectively provides immune protection for diseases. Preparation of the divalent vaccine is low in cost of used materials and safe to animals and the environment.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Bi-recombinant active carrier vaccine of co-expression pig gyrate virus and foot and mouth disease virus
InactiveCN1749397AEffective immunityNo pathologySugar derivativesGenetic material ingredientsCircovirusCell immunity
The present invention belongs to the field of biotechnology, and is especially one kind of recombinant live carrier vaccine co-expressing pig gyrate virus and foot and mouth disease virus. The present invention aims at providing two recombinant chicken pox viruses to co-express capsid protein ORF2 or its fusion protein ORF2-ORF1 of the China epidemic strain of type-II pig gyrate virus and the structural protein precursor P1 of the China epidemic strain NY00 of type-O foot and mouth disease virus, and for serving as the duplex live carrier gene engineering vaccine to prevent PCV2 and FMDV infection. The two recombinant chicken pox viruses can stimulate mouse to generate effective body fluid immunity and cell immunity, and are safe to experimental animals without any pathological phenomenon.
Owner:金宁一
Meningococcus antigen combination and application thereof
ActiveCN104072590AEffective immunityActivate immune responseAntibacterial agentsBacteriaAntigenMeningococcal carriage
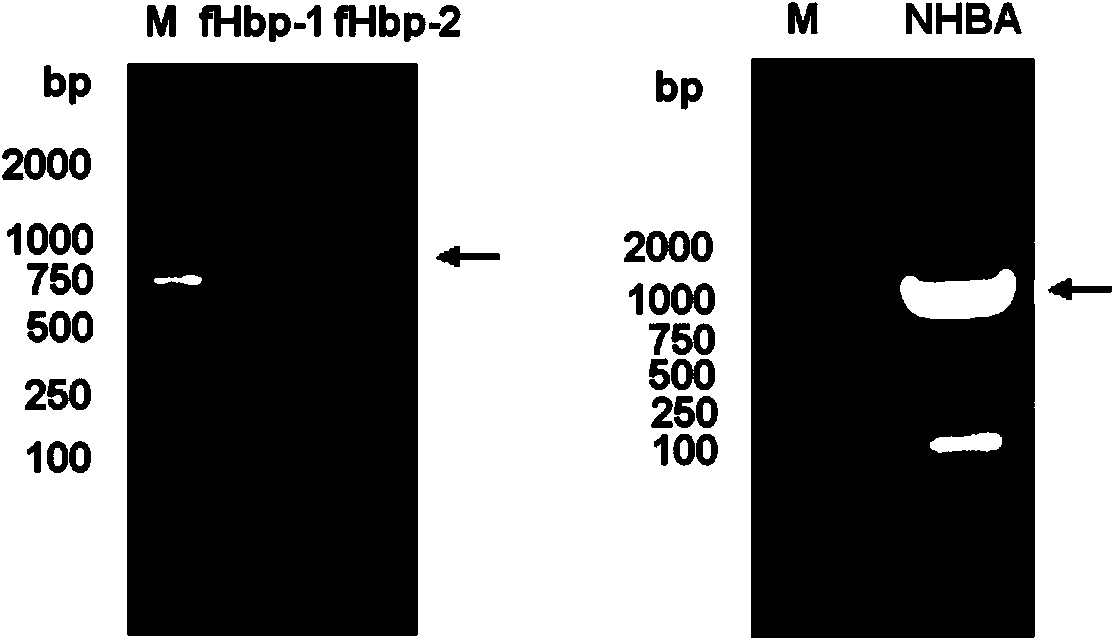
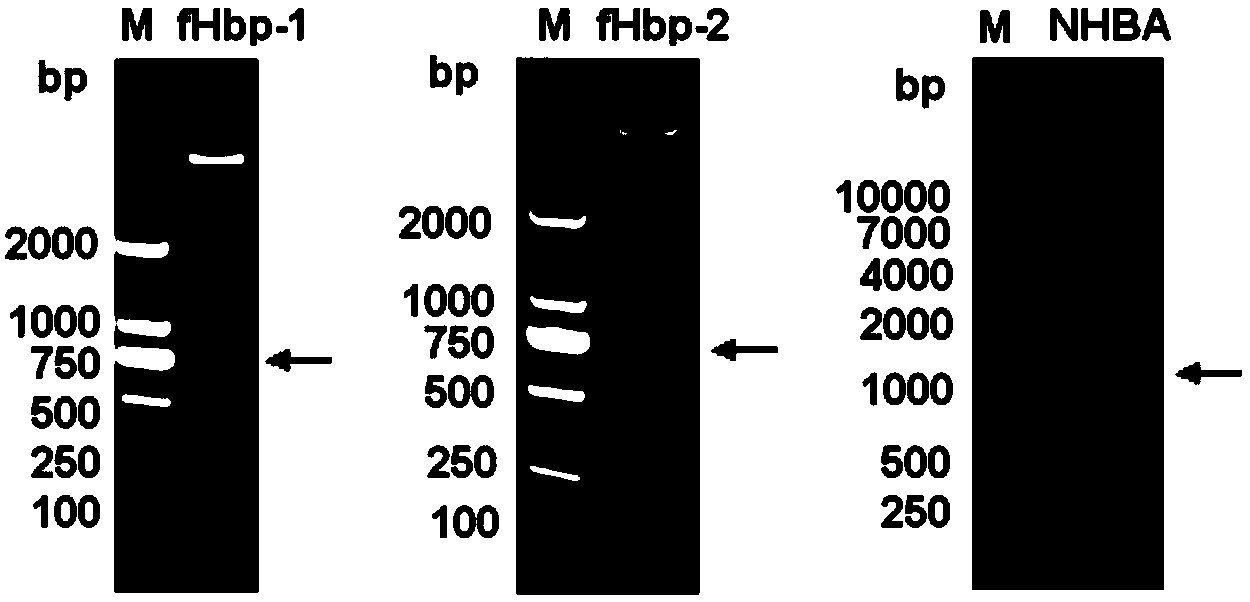
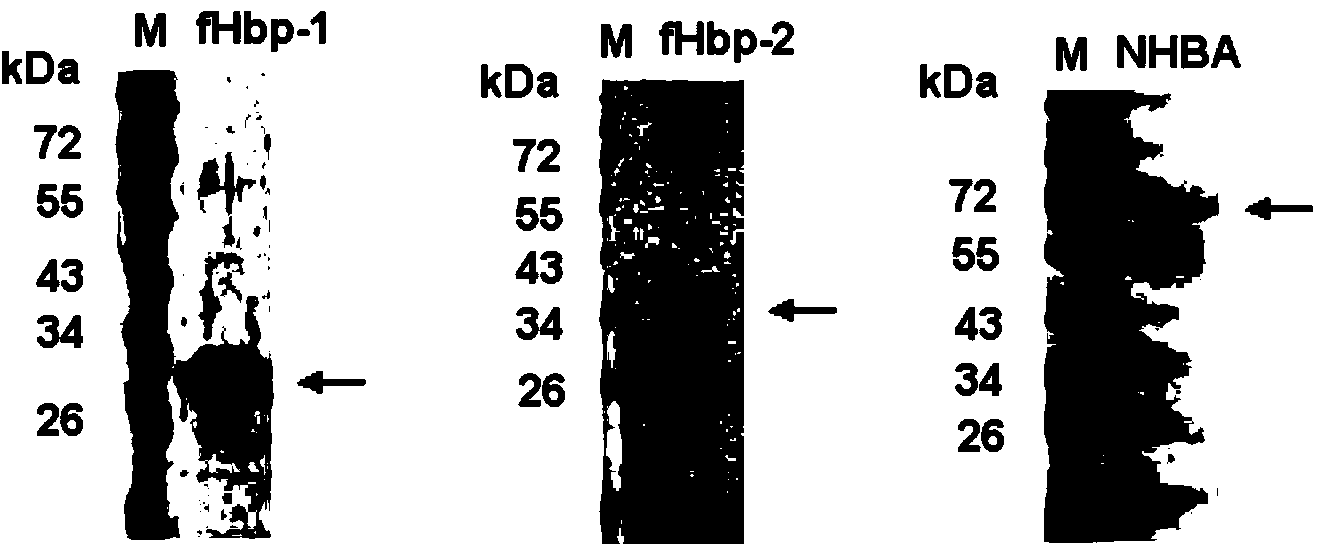
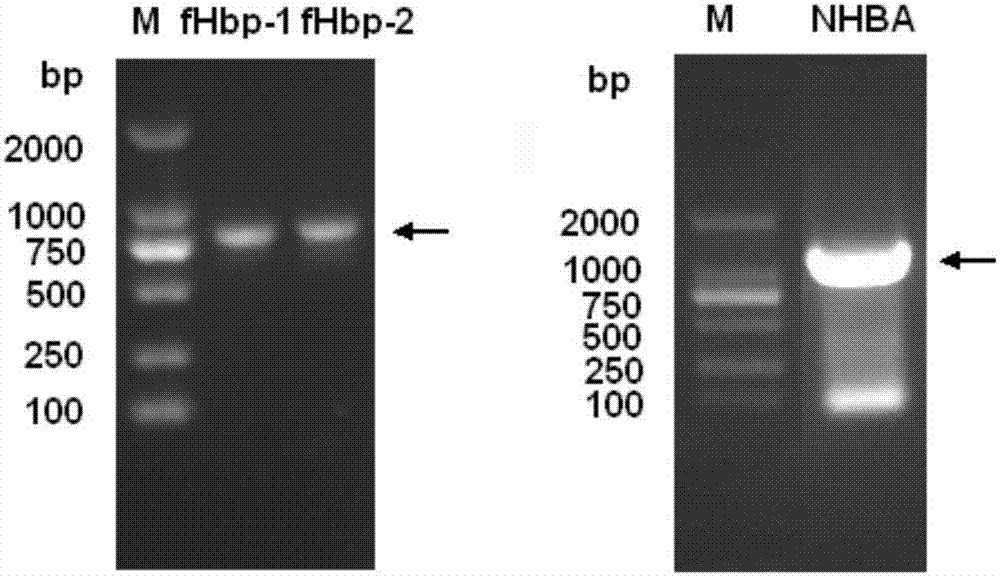
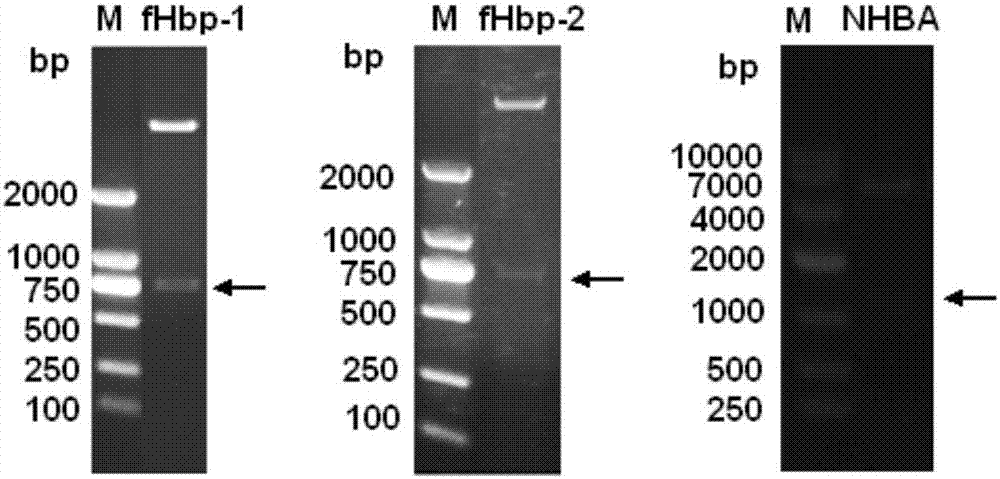
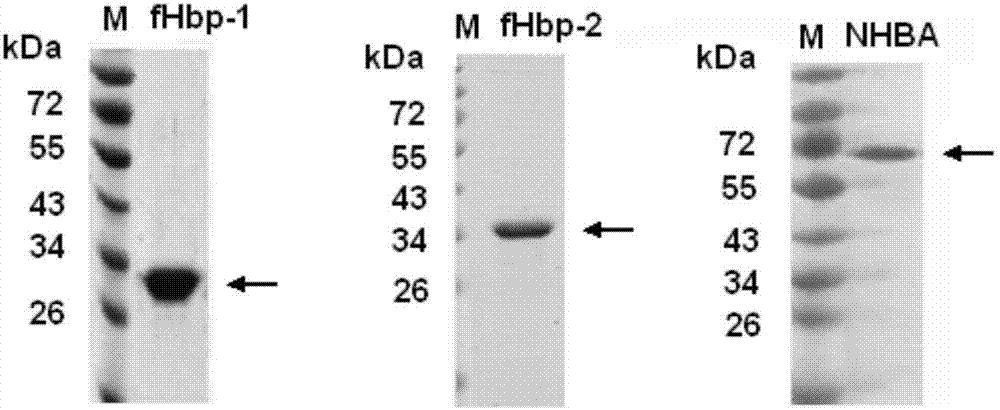
The invention provides a novel antigen combination of meningococcus and an application thereof. The antigen combination comprises fHbp V1 variant proteins, fHbp V2 variant proteins and NHBA proteins. The experiment shows that vaccines prepared from the antigen combination have an obvious synergistic effect and have high broad-spectrum meningococcus resistant capability.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Recombinant attenuated salmonella typhimurium vector vaccine expressing PCV-2 immunogenic gene and preparation method thereof
InactiveCN101954074AImproving immunogenicityImprove protectionBacteriaViral antigen ingredientsDiseaseVector vaccine
The invention discloses an oral recombinant attenuated salmonella typhimurium live vector vaccine as well as a preparation method and application thereof, particularly relating to an oral attenuated salmonella typhimurium. The recombinant attenuated salmonella typhimurium has a sequence shown in SEQ ID NO.1, and can express PCV-2 capsid protein ORF2. The attenuated salmonella typhimurium is inoculated in an LB culture medium for culturing for 18 hours; and the concentration of the attenuated salmonella typhimurium is regulated to 1010CFU / ml to prepare a safe and effective oral attenuated salmonella typhimurium live vector vaccine used for preventing porcine circovirus diseases.
Owner:NORTHWEST A & F UNIV
Fusion protein sHA1-Fc and application
InactiveCN106117369AProtect animal organismsSimple and efficient operationSsRNA viruses negative-senseAntibody mimetics/scaffoldsSpecific immunityFc fragment
The invention discloses fusion protein sHA1-Fc and an application. The fusion protein sHA1-Fc of a highly pathogenic AIV (avian influenza virus) H5N1 and a chicken IgY Fc fragment is expressed by a Bacto-Bac expression system, has a molecular structure similar to IgY, and can penetrate a respiratory tract mucosal barrier of a chicken to enter the body under transfer of FcRy expressed in epithelial cells. A sequence of an sHA1-Fc fusion gene is represented as SEQ ID NO:1. The fusion protein is supplemented with a mucosal adjuvant and inoculates the chicken through nasal inhalation, thereby being capable of triggering an organism to generate effective mucosal immunity and triggering systemic immunity of the organism. Therefore, the organism is enabled to obtain anti-AIV local specific immunity and systemic immunity, the immune effect of the fusion protein is better than that of a commercial inactivated virus vaccine, and a new strategy is provided for development of a novel mucosal vaccine for poultry bird flu and other infectious diseases.
Owner:HUAZHONG AGRI UNIV
Recombinant attenuation salmonella typhimurium vector vaccine expressing PRRSV (Porcine Reproductive and Respiratory Syndrome Virus) immunogen gene and preparation method thereof
InactiveCN101920010AImproving immunogenicityImprove protectionBacteriaViral antigen ingredientsBacteroidesCyst
The invention discloses a recombinant attenuation salmonella typhimurium vector vaccine expressing a PRRSV (Porcine Reproductive and Respiratory Syndrome Virus) immunogen gene and a preparation method and application thereof, and in particular relates to an oral attenuation salmonella typhimurium. The recombinant attenuation salmonella typhimurium has a sequence shown in SEQ ID NO.1, and can express PRRSV cyst membrane protein GP5. The attenuation salmonella typhimurium is inoculated in an LB (Lysogeny Broth) culture medium and cultured for 18h, and the bacterium concentration is regulated to be 1010CFU / ml, therefore, the safe and effective oral attenuation salmonella typhimurium live vector vaccine is prepared and used for preventing PRRS.
Owner:NORTHWEST A & F UNIV
Freeze-drying slow-release protective agent used for preparing adenovirus preparation and adenovirus preparation and preparation method thereof
InactiveCN108175774AEfficient transferReduce pathogenicityPowder deliveryViral antigen ingredientsOral medicationFreeze-drying
The invention discloses a freeze-drying slow-release protective agent used for preparing an adenovirus preparation and the adenovirus preparation and a preparation method thereof, which relate to thefield of an adenovirus preparation. The freeze-drying slow-release protective agent comprises a first solution containing sodium alginate and a second solution containing calcium chloride, the freeze-drying slow-release protective agent solves the problem of stability of an oral adenovirus preparation, has enteric characteristic, and can protect adenovirus from influence of gastric acid, and the preparation can keep stabilization under low-pH stomach environment, after entering into intestinal tract, the preparation is gradually disintegrated and releases adenovirus, and the adenovirus preparation is suitable for an oral administration mode, which overcomes inconvenience of an injection administration mode. The problem of easy deactivation of preservation of the adenovirus liquid preparation is solved, preservation time of the preparation is prolonged, the requirement of the adenovirus preparation on temperature during preservation and transport processes can be reduced, preparation stability is increased, and economic cost is reduced.
Owner:CHENGDU YUANRUI BIOTECH CO LTD
Method for inducing early t memory response with short peptides Anti-tumor vaccine
ActiveUS20180169200A1Large capacityEffective immunityCancer antigen ingredientsAntigen/adjuvant combination ingredientsPeptide TWhite blood cell
The present invention relates to a therapeutic peptide T specific immune therapy for use in the treatment of a cancer of an HLA-A2 (Human Leukocyte Antigen A2) positive patient, said treatment comprises a priming period consisting in two to three administrations of said therapeutic peptide T specific immune therapy, thereby inducing a memory T cell response.
Owner:OSE IMMUNOTHERAPEUTICS
Recombinant baculovirus expressing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) immunogen gene and preparation method and application thereof
InactiveCN101792740AAvoid the tedious process of separation and purificationEasy to measureViral antigen ingredientsGenetic material ingredientsFreeze thawingPorcine reproductive and respiratory syndrome virus
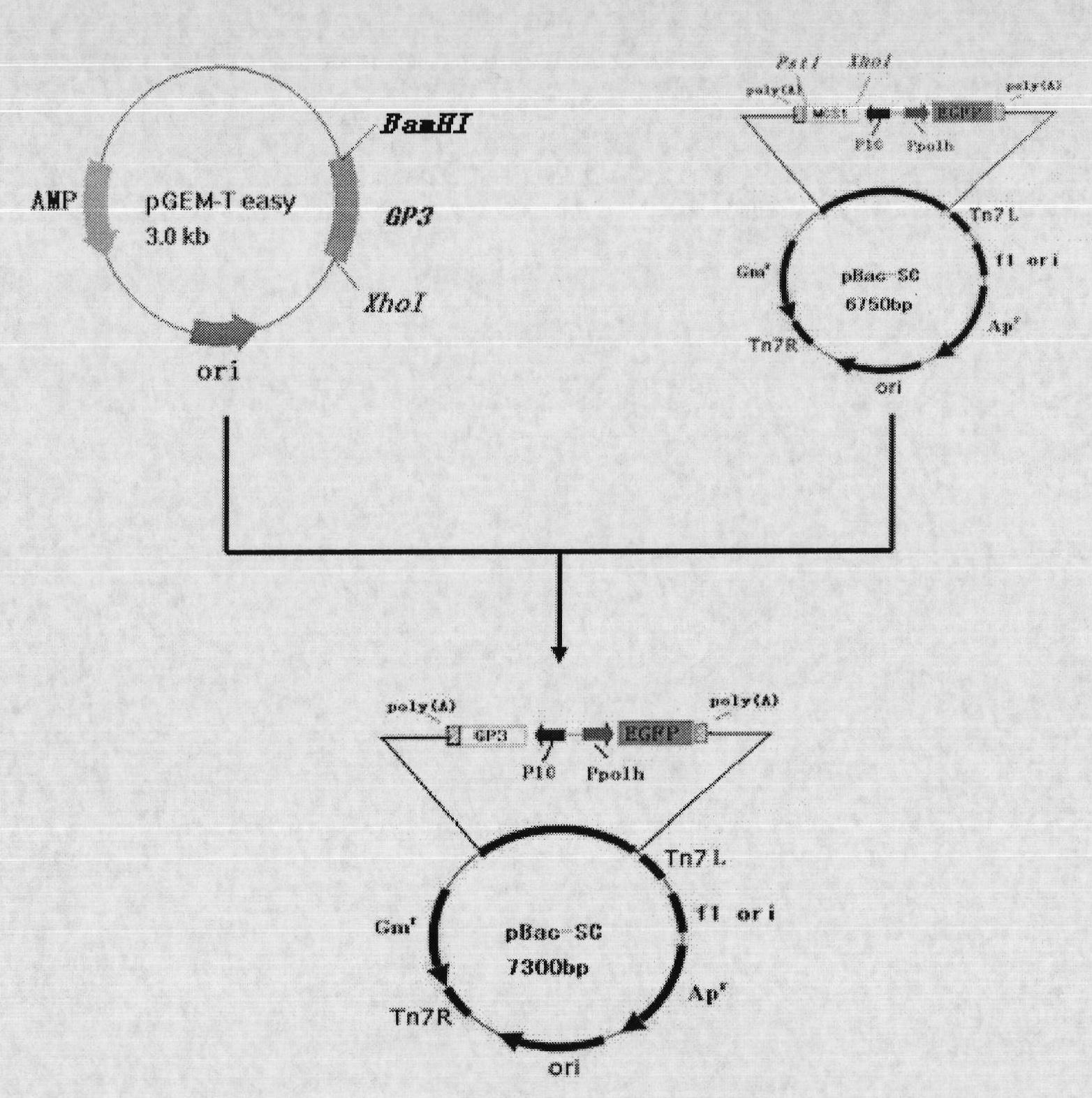
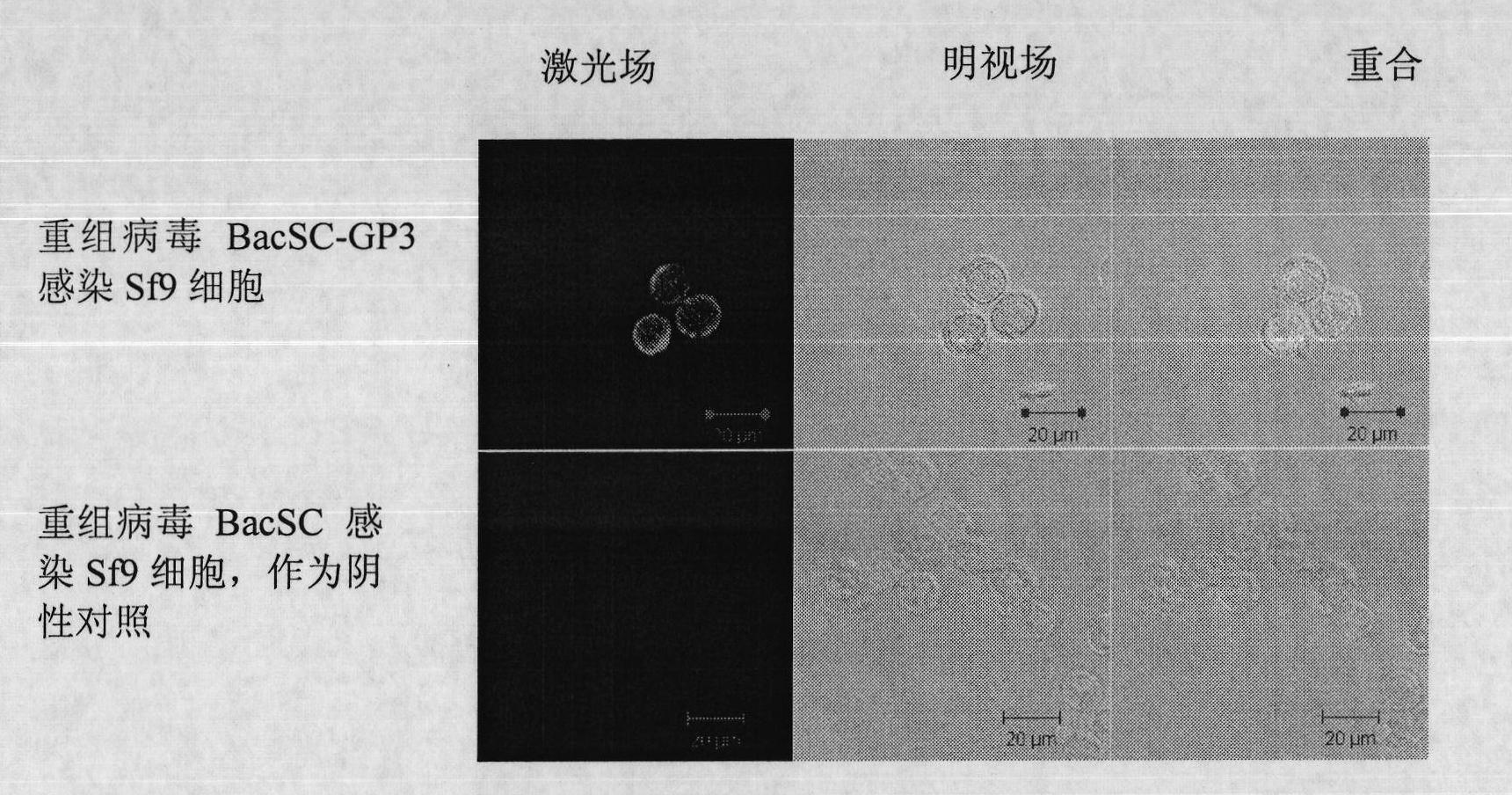
The invention discloses a recombinant baculovirus expressing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) immunogen genes and a preparation method and application thereof, particularly relates to a recombinant baculovirus comprising a sequence shown as SEQ ID NO.1 and displaying structural proteins GP3 of the PRRSV on the surface. The preparation method comprises steps of utilizing the recombinant baculovirus to inoculate insect cells, culturing for 48-72h, collecting infection cells, carrying out freeze thawing at 40DEG C below zero / 37DEG C, centrifuging, taking supernate to test virus PFU and adjusting the titer of the virus to 109PFU / ml to obtain a safe and effective gene engineered subunit vaccine for preventing PRRS.
Owner:NORTHWEST A & F UNIV
Attenuated ehrlichiosis vaccine
ActiveUS8361480B2Avoid problemsEffective immunityAntibacterial agentsBacterial antigen ingredientsEhrlichia canisPathogenicity
The present invention relates to an attenuated strain of Ehrlichia canis and a vaccine comprising said attenuated strain for protection of mammals against ehrlichiosis. The invention further relates to methods of preventing ehrlichiosis and of attenuating the pathogenicity Ehrlichia canis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
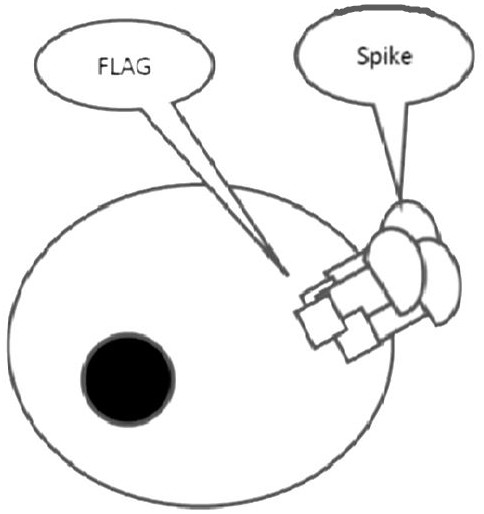
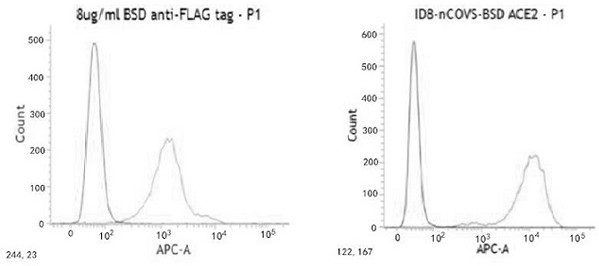
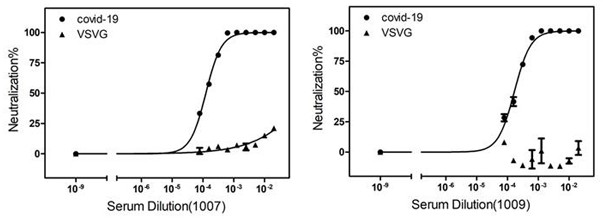
2019-nCoV S-antigens for generating 2019-nCoV neutralizing antibodies and method for preparing 2019-nCoV S-antigens
InactiveCN113248581AImprove immunityEffective immunitySsRNA viruses positive-senseSerum immunoglobulinsAntigenCell membrane
The invention discloses a 2019-nCoV S-antigens for generating 2019-nCoV neutralizing antibodies and a method for preparing the 2019-nCoV S-antigens. According to the 2019-nCoV S-antigens and the method, the 2019-nCoV S-antigens comprise 2019-nCoV S-antigen trimers combined with a cell membrane based on a Flag tag. The 2019-nCoV S-antigens can generate high-titer neutralizing antibodies so as to more effectively immunize animals.
Owner:江西浩然生物制药有限公司
A kind of porcine epidemic diarrhea recombinant baculovirus genetically engineered subunit vaccine and its preparation method and application
InactiveCN103585625BImprove abilitiesPracticalMicroorganism based processesAntiviralsElisa kitEngineered genetic
The invention belongs to the technical field of biological vaccine preparation, in particular to a porcine epidemic diarrhea recombinant baculovirus genetically engineered subunit vaccine and its preparation method and application. The present invention selects the S1 gene and M gene of the current new epidemic strain of PEDV as the reference sequence, uses the baculovirus expression system to express the S1 protein or part of the S1 protein and M protein, and prepares the obtained recombinant protein into a subunit vaccine for effective Control the occurrence of porcine epidemic diarrhea. The porcine epidemic diarrhea genetically engineered subunit vaccine prepared by the method of the present invention solves the defects of the current porcine epidemic diarrhea virus traditional vaccine, can be used to prevent and treat porcine epidemic diarrhea virus infection and related diseases caused by it, and can also It is also suitable for preparing the coating antigen of the ELISA kit for detecting porcine epidemic diarrhea virus antibody.
Owner:SOUTH CHINA AGRI UNIV
Subunit vaccine for porcine reproductive and respiratory syndrome as well as preparation method and application of subunit vaccine
ActiveCN111978411ASimple purification processEasy to separate and purifySsRNA viruses positive-senseVirus peptidesGenetic engineeringCellular immunity
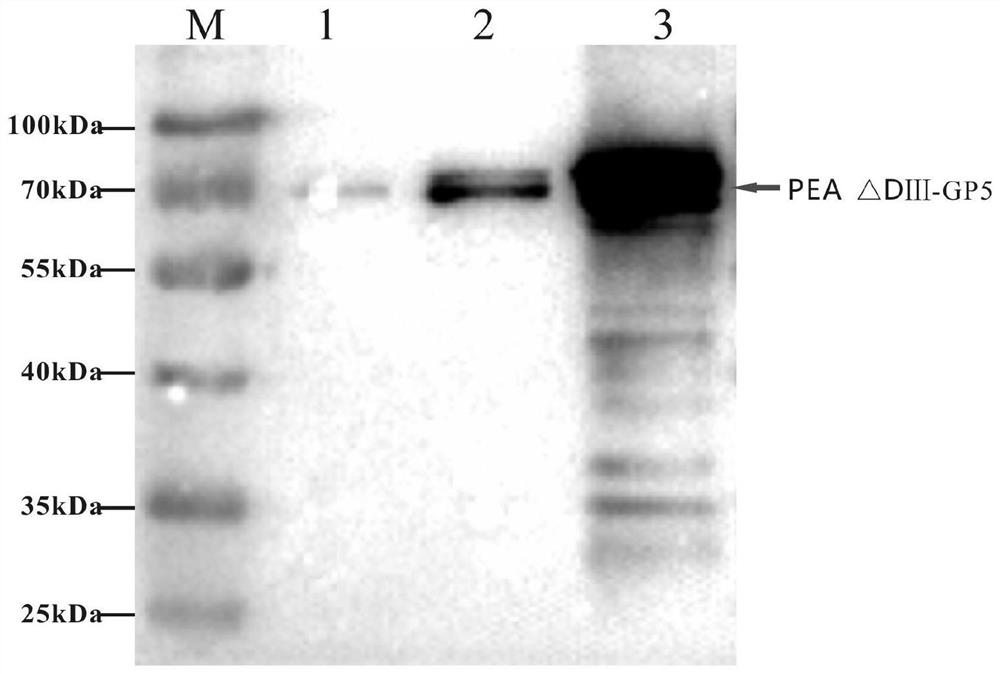
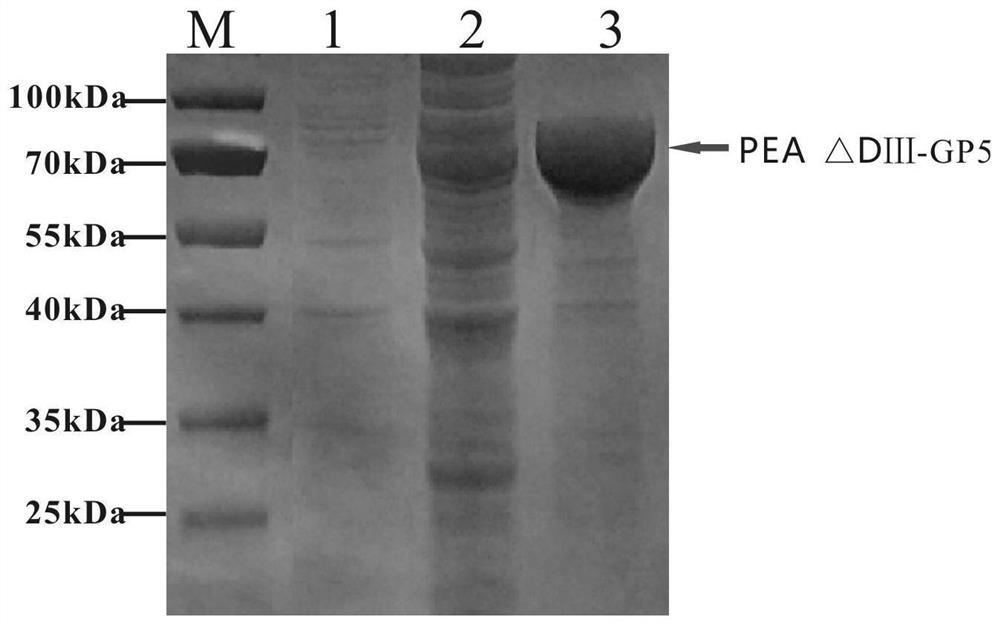
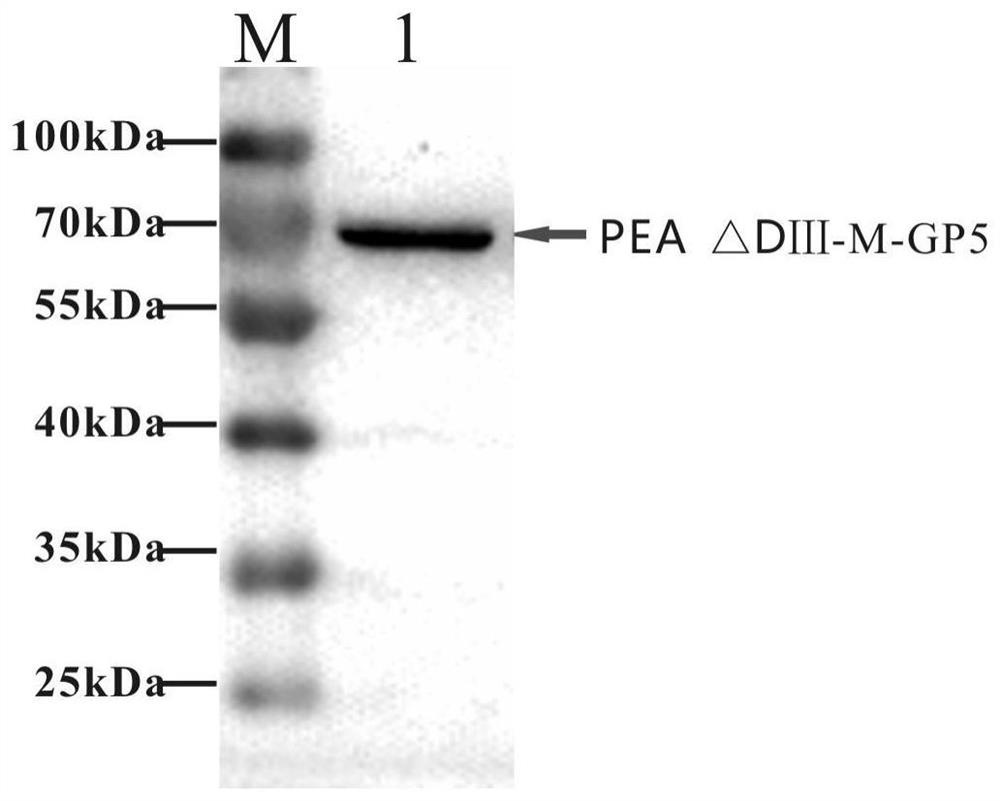
The invention relates to the fields of genetic engineering and veterinary biological pharmacy, and in particular discloses a subunit vaccine for porcine reproductive and respiratory syndrome as well as a preparation method and application of the subunit vaccine. The invention firstly provides a fusion protein, wherein an amino acid sequence of the fusion protein is as shown in SEQ ID No. 1; and then the invention provides the subunit vaccine for the porcine reproductive and respiratory syndrome, wherein the subunit vaccine comprises the fusion protein. The fusion protein in the subunit vaccinefor the porcine reproductive and respiratory syndrome is obtained by conducting fusion expression on an ORF5 full-length gene of a PRRSV NADC30-like strain, which is optimized by virtue of a codon, and a gene of pseudomonas aeruginosa, from which a Domain III structural Domain is removed, and by virtue of an insect baculovirus / insect cell expression system. The vaccine provided by the inventionis good in protective effect, capable of inducing effective humoral immunity and cellular immunity and is suitable for clinical prevention of the reproductive and respiratory syndrome.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
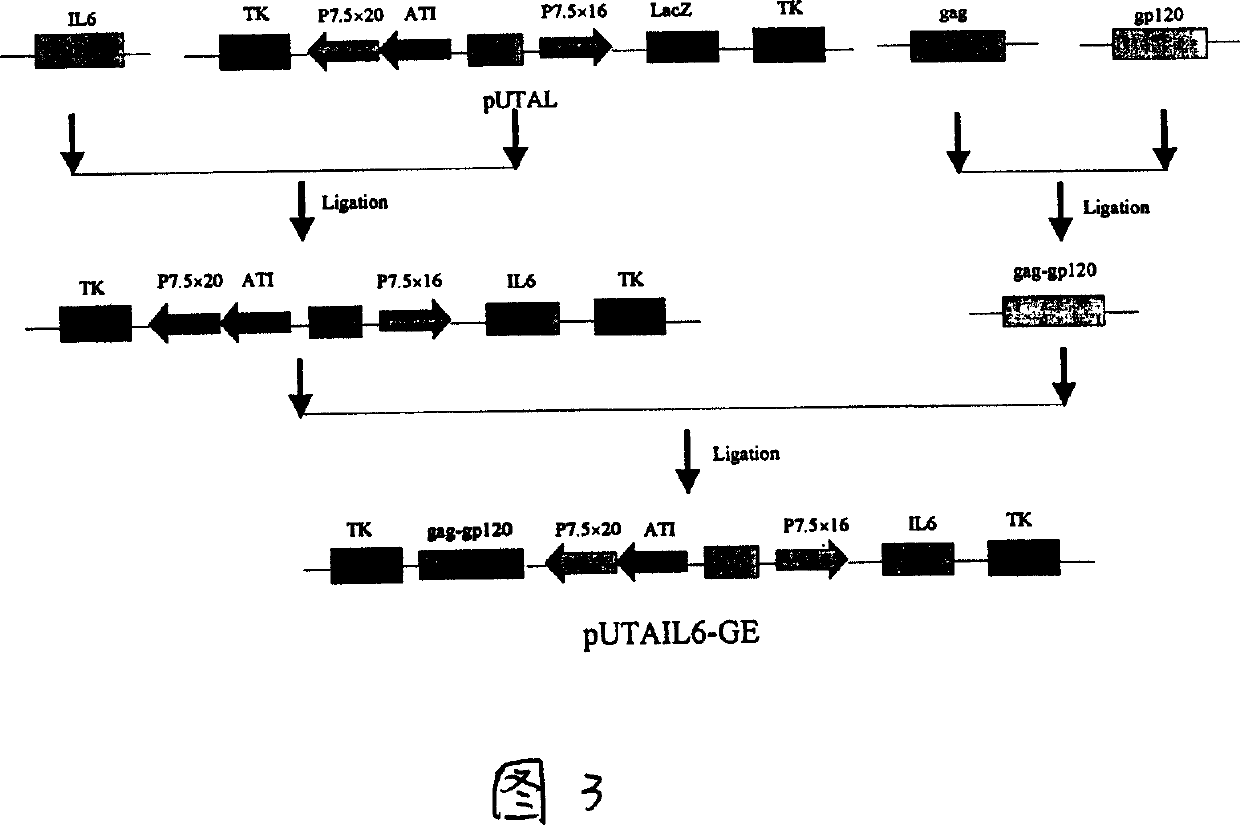
Recombined fowl pox living carrier vaccine for co-expression of MIV-1CNB and hlL-6 proteins
InactiveCN1490052AGood genetic stabilityEffective immunityAntiviralsAntibody medical ingredientsLive vector vaccineProtein A
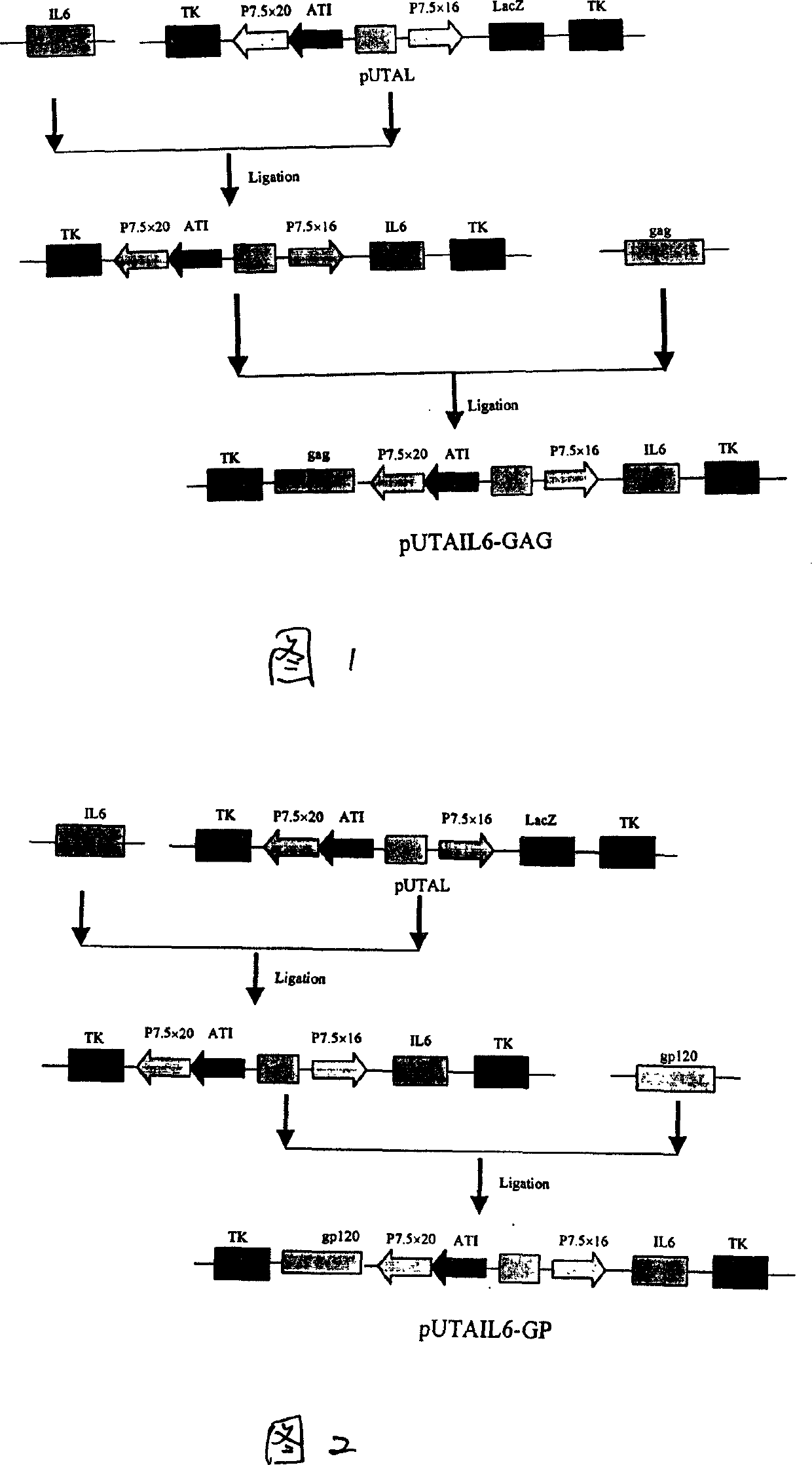
A recombinant live-carrier vaccine for henpox is disclosed, which can express the structural protein HIV-1CNB and hIL-6. Three recombinant henpox virus strains vPUTAIL6-GAG, vPUTAIL6-GP and vPUTAIL6-GE can express the HIV-1CNB core protein gag, outer membrane protein gp120 or chimeric protein gap-gp120, and hIL-6. They can stimulate the organ to generate HIV-1 resistant specific homoral immune and cell immune. Its advantages are high safety and high genetic stability.
Owner:MILITARY VETERINARY INST MILITARY SUPPIES PLA
False virosome vaccine with recombinant replicon of dengue fever virus as carrier
InactiveCN1524952AImprove effectivenessEffective immunityViral antigen ingredientsViral/bacteriophage medical ingredientsDendritic cellDengue virus
The invention provides a false virus granule vaccine with dengue fever virus recombination replicon as a core and method for preparation, wherein the vaccine can express antigens with high efficiency in the affected cells, the antigen can be extracted effectively with good immunity effect. The vaccine can be used for preventing and treating tumor and virosis.
Owner:上海天甲生物医药有限公司 +2
Meningococcus antigen composition and applications thereof
ActiveCN107349423AEffective immunityActivate immune responseAntibacterial agentsPeptidesAntigenMutated protein
The invention provides a novel meningococcus antigen composition and applications thereof. Specifically, the antigen composition comprises fHbp V1 mutant protein, fHbp V2 mutant protein, and NHBA protein. The experiment results show that a vaccine prepared from the antigen composition has a prominent synergistic effect and an excellent performance on resisting meningitis in a broad spectrum.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com