Meningococcus antigen combination and application thereof
An antigen and vaccine composition technology, applied in the field of vaccines, can solve the problems of increasing the titer and side effects of vaccines and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 Construction of Recombinant Expression Engineering Bacteria
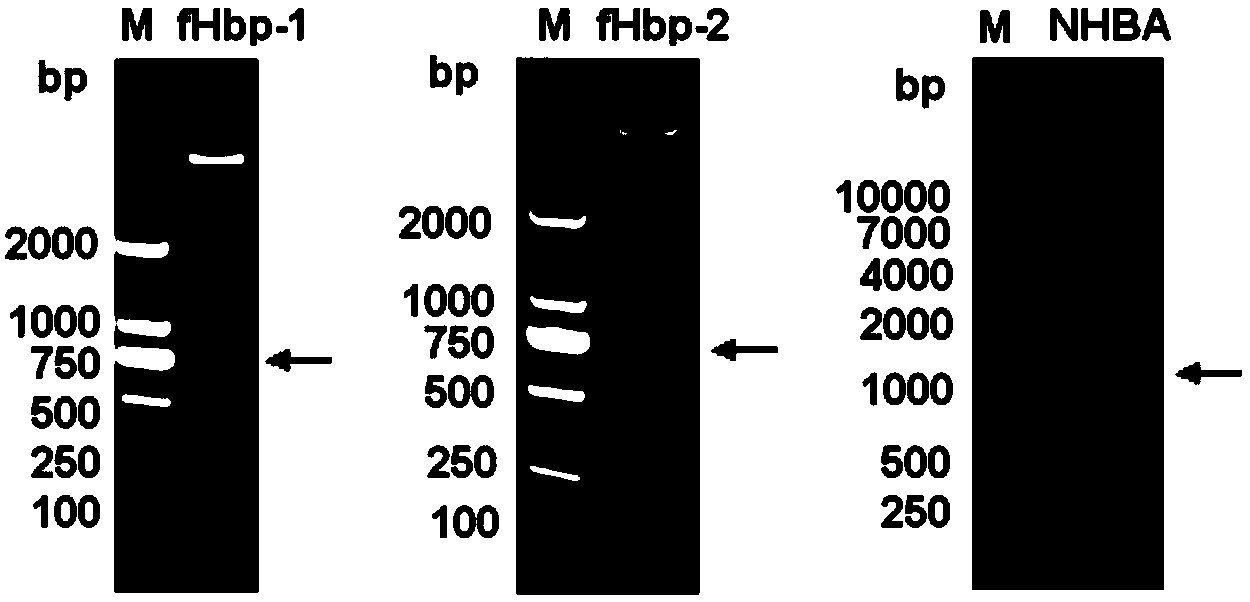
[0095] Genomes were extracted from the cultures of three clinical isolates of meningococcus, and the first fHbp (named fHbp-1, ie variant 1) and the second fHbp ( Named as fHbp-2, namely variant 2) and NHBA gene. PCR amplification forward primer and reverse primer are listed in the table below, and forward primer and reverse primer introduce the restriction enzyme cutting site of Nde I and BamH I (fHbp) or Nhe I and BamH I (NHBA) respectively, with are underlined. The recombinant fHbp and NHBA proteins were expressed in Escherichia coli in the form of His-tag fusion, and the plasmid pET-28a (Novagen) was used as the expression vector.
[0096]
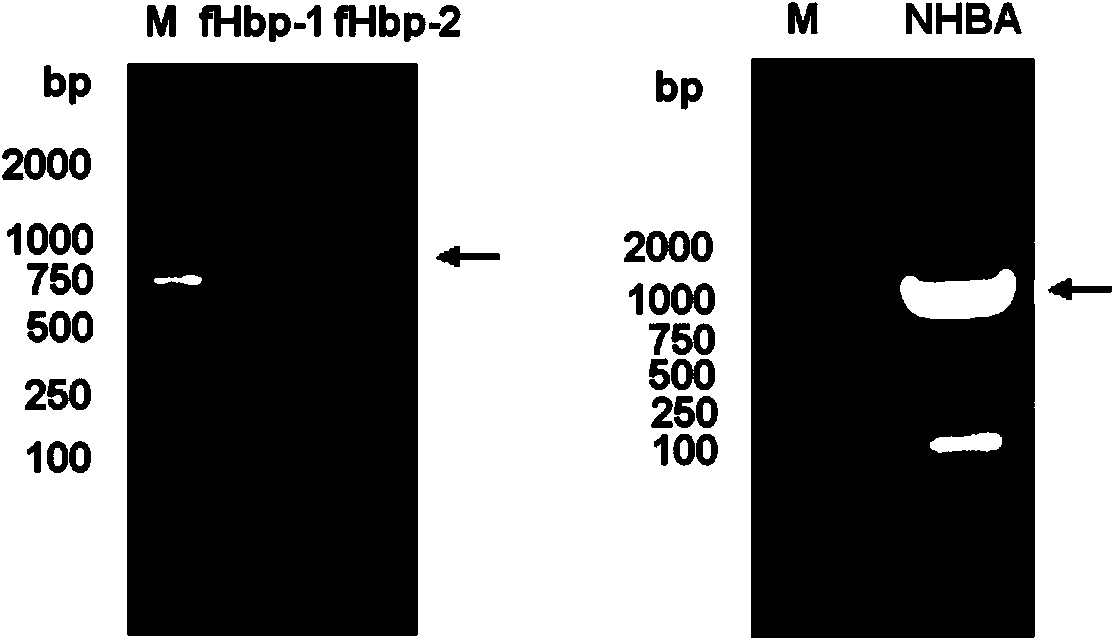
[0097] Cloning of fHbp-1 and fHbp-2: PCR was performed from bacterial genomic DNA using rTaq DNA polymerase (TaKaRa). The PCR reaction conditions were: 95°C for 5 min; 35 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min; 72°C for 8 min. Th...
Embodiment 2
[0101] Embodiment 2 fermentation culture
[0102] Fermentation of recombinant fHbp-1 and fHbp-2. Transform the recombinant plasmid into BL21 (DE3) competent, pick the monoclonal colony on the plate, inoculate it into 5ml LB liquid medium containing kanamycin, culture it with shaking at 37°C overnight, and transfer it to 250ml kanamycin-containing medium the next day In the Erlenmeyer flask of the LB liquid medium of mycin, shake culture at 37°C until the absorbance value of 600nm is A 600 value is 0.6, add IPTG to a final concentration of 0.6mmol / L, continue shaking culture for 5 hours, centrifuge at 6000rpm for 10 minutes, and collect the bacteria.
[0103] Fermentation of recombinant NHBA. Transform the recombinant plasmid into BL21 (DE3) competent, pick the monoclonal colony on the plate, inoculate it into 5ml LB liquid medium containing kanamycin, culture it with shaking at 37°C overnight, and transfer it to 250ml kanamycin-containing medium the next day In the Erlenmey...
Embodiment 3
[0104] Example 3 Purification of target protein
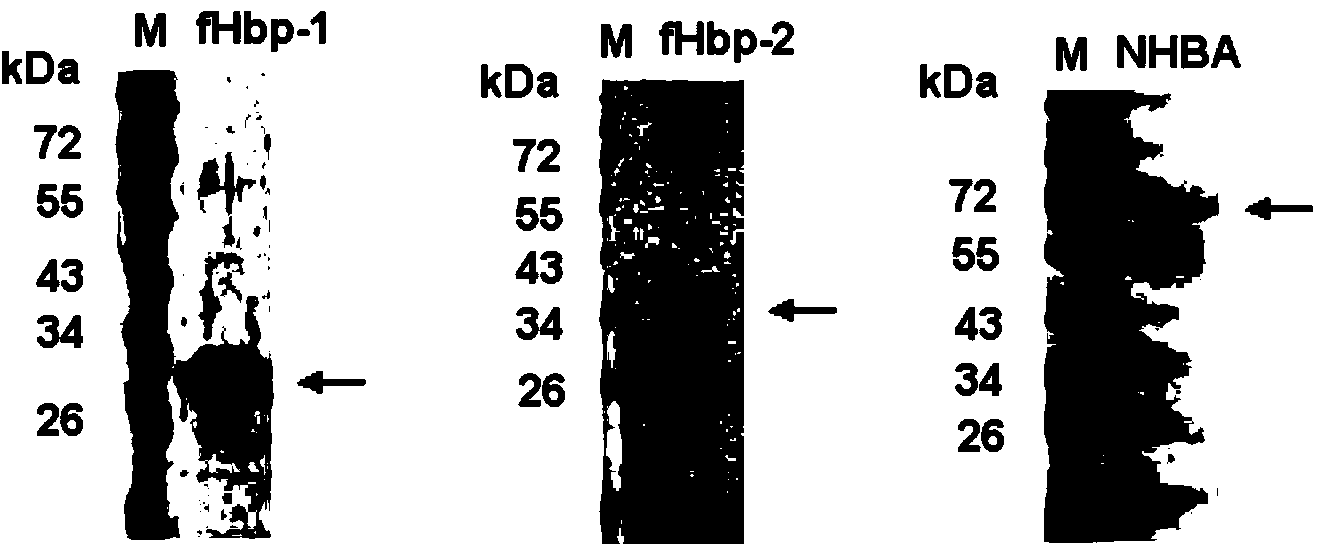
[0105] Resuspend the bacterial body fluid with nickel column equilibration buffer and place it in ice-water mixture, and ultrasonically break the bacteria. After breaking the bacteria, the supernatant was harvested by centrifugation. Ni 2+ The crude product was purified by affinity chromatography column, the recombinant fHbp-1 and fHbp-2 were further purified by gel filtration chromatography, and the recombinant NHBA was further purified by CM ion column. The SDS-PAGE electrophoresis patterns of purified recombinant fHbp-1, fHbp-2 and NHBA proteins are shown in Figure 4 . The purified protein was stored at -20°C for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com