Integrated methods for chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
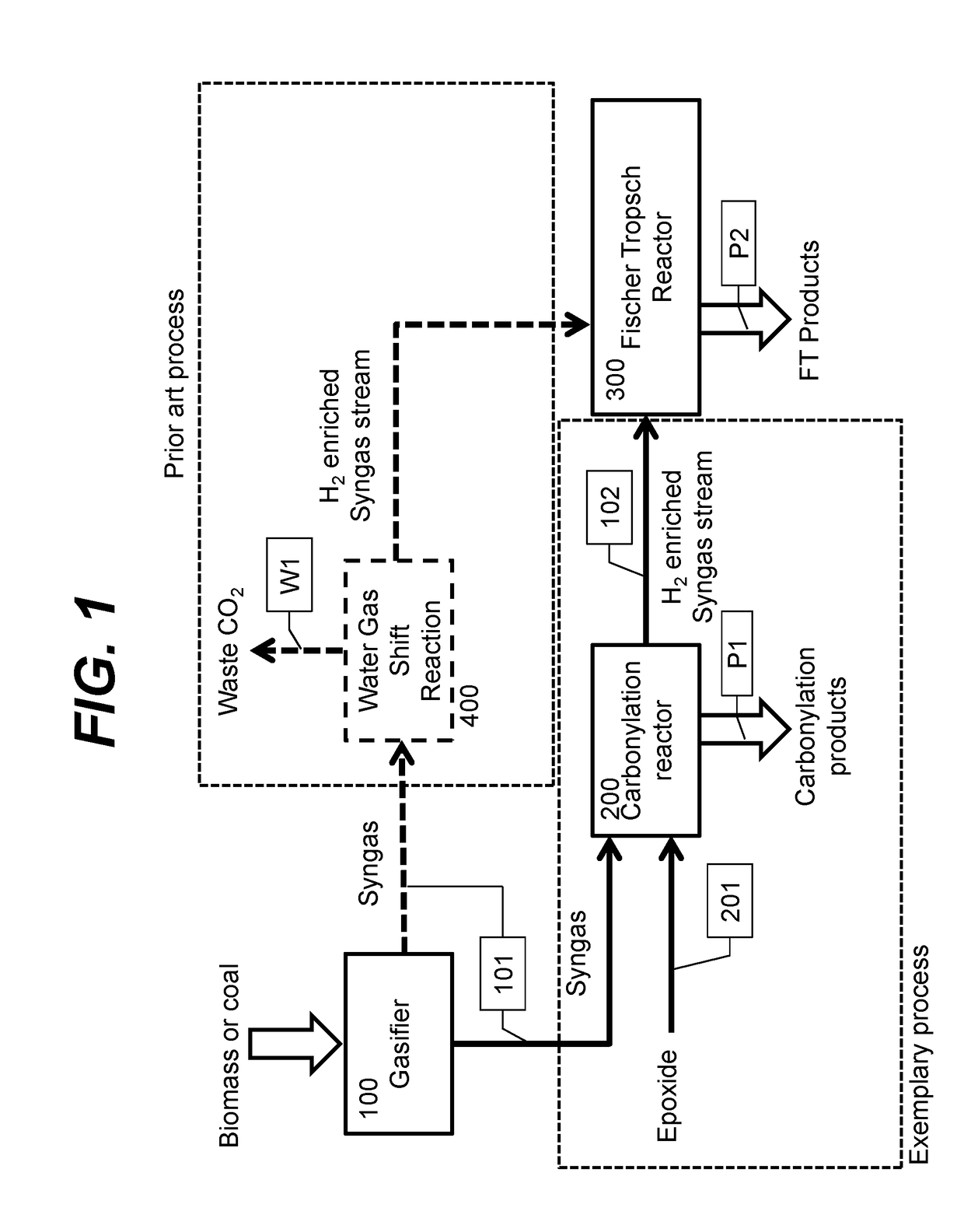
2. The method of embodiment 1, wherein the syngas stream has a molar hydrogen to CO ratio between 0.5:1 and 1.2:1 and the upgraded gas stream has a hydrogen to CO ratio of at least 1.9:1.
3. The method of embodiment 1, wherein the second chemical process comprises Fischer Tropsch synthesis.
4. The method of embodiment 1, wherein the second chemical process comprises reaction on a fuel cell.
5. The method of embodiment 1, wherein the second chemical process comprises hydrogenation.
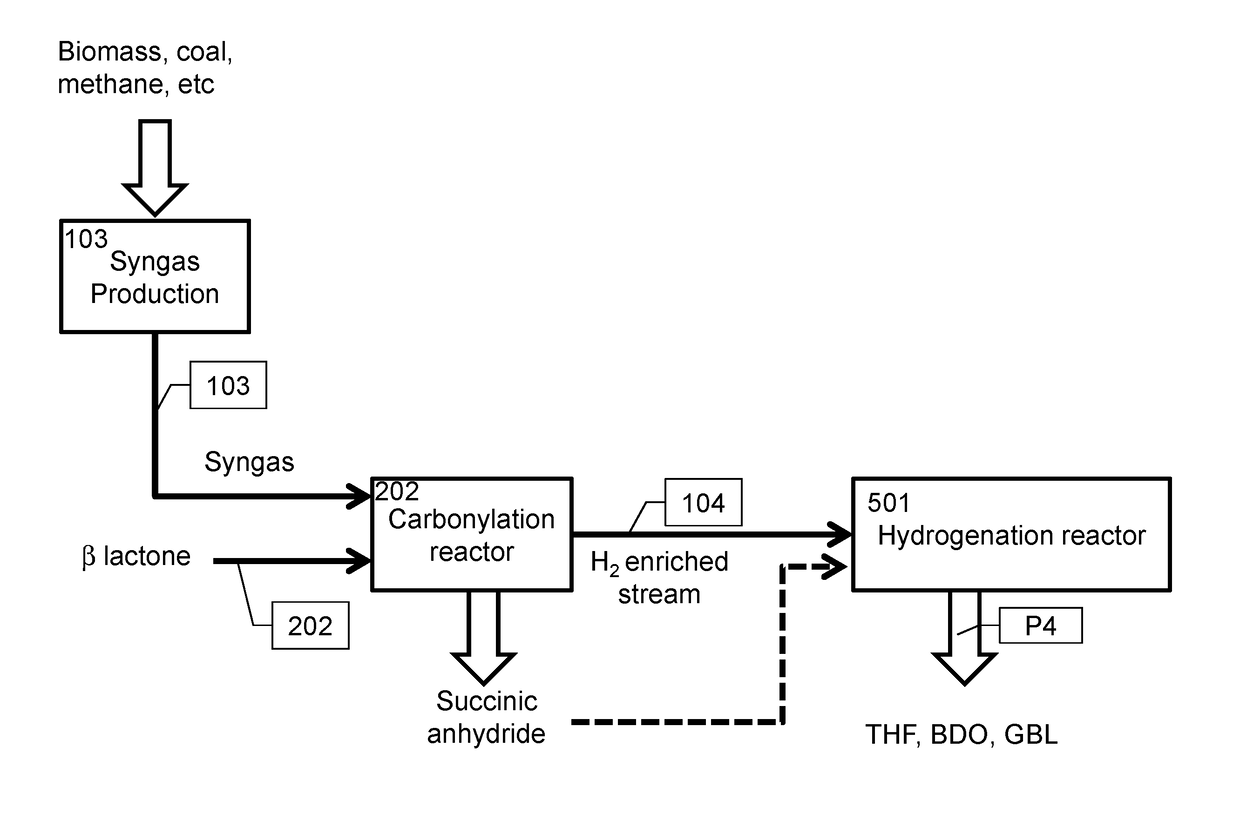
6. The method of embodiment 1, wherein the epoxide carbonylation product is selected from the group consisting of: optionally substituted beta lactone, optionally substituted succinic anhydride, and a polyester resulting from alternating polymerization of CO and the epoxide.
7. The method of embodiment 1, wherein the epoxide is ethylene oxide.
embodiment 7
8. The method of embodiment 7, wherein the carbonylation product is beta propiolactone.
9. The method of embodiment 7, wherein the epoxide carbonylation product is succinic anhydride and the upgraded gas stream has a molar hydrogen to CO ratio greater than 5:1.
embodiment 9
10. The method of embodiment 9, wherein and the upgraded gas stream has a hydrogen to CO ratio greater than 10:1, greater than 20:1, greater than 50:1, greater than 100:1, or greater than 1,000:1.
11. The method of embodiment 9, wherein the upgraded gas stream is substantially free of carbon monoxide.
12. Beta-propiolactone produced by carbonylation of ethylene oxide having a pMC of zero, as defined by ASTM D6866, using carbon monoxide having a pMC greater than zero, as defined by ASTM D6866.
13. Beta-propiolactone produced by carbonylation of ethylene oxide having a pMC greater than zero, as defined by ASTM D6866, using carbon monoxide having a pMC of zero, as defined by ASTM D6866.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com