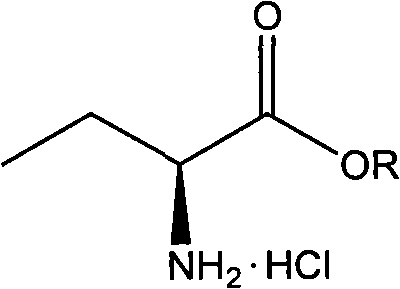
Novel method for preparing levetiracetam midbody S-(+)-2-aminobutyrate hydrochlorate
A technology of aminobutyrate and aminobutyric acid, which is applied in the field of preparing levetiracetam intermediate S--2-aminobutyrate hydrochloride, can solve the problems of no literature reports, etc., and achieve simple steps and technical Parameters are easy to control the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Suspend 20.6 grams (0.2 moles) of 2-aminobutyric acid in 200 milliliters of ethanol, add 26 grams of thionyl chloride dropwise within 1 to 1.5 hours under stirring at 0 to 7 ° C to form a clear solution, stir at room temperature for about After 15 hours, the reaction solution was concentrated to dryness under reduced pressure, then ammonia water was added, and the oil layer was separated to obtain 25.5 g of ethyl DL-2-aminobutyrate with a yield of 97%.
[0036] In a 500 ml flask, add 13.1 g of ethyl 2-aminobutyrate and 25 ml of 95% ethanol. Heat to 80°C, add 6.7 g of L-malic acid, stir for 2 hours, filter, wash with an appropriate amount of 95% ethanol, the filter cake is S-(+)-2-aminobutyric acid methyl ester L malate solid. Add ethyl acetate to the solid of S-(+)-2-aminobutyric acid ethyl ester L-malate, feed ammonia gas for 2 hours, filter, wash with ethyl acetate, dry, and recover the solvent to obtain S-(+ )-2-aminobutyric acid ethyl ester 5.8 grams. Yield 44%, p...
Embodiment 2
[0040] Suspend 20.6 grams (0.2 moles) of 2-aminobutyric acid in 200 milliliters of ethanol, add 26 grams of thionyl chloride dropwise within 1 to 1.5 hours under stirring at 0 to 7 ° C to form a clear solution, stir at room temperature for about After 15 hours, the reaction solution was concentrated to dryness under reduced pressure, then ammonia water was added, and the oil layer was separated to obtain 25.5 g of ethyl DL-2-aminobutyrate with a yield of 97%.
[0041] In a 500 ml flask, add 13.1 g of ethyl 2-aminobutyrate and 25 ml of 95% ethanol. Heat to 35°C, add 6.7 g of L-malic acid, stir for 3 hours, filter, wash with an appropriate amount of 95% ethanol, the filter cake is S-(+)-2-aminobutyric acid methyl ester L malate solid. Add ethyl acetate to the solid of S-(+)-2-aminobutyric acid ethyl ester L-malate, feed ammonia gas for 3 hours, filter, wash with ethyl acetate, dry, and recover the solvent to obtain S-(+ )-2-aminobutyric acid ethyl ester 5.67 grams. Yield 43%, pu...
Embodiment 3
[0045] Under stirring at 0-7°C, 103 grams (1.0 mole) of 2-aminobutyric acid was put into 250 milliliters of methanol, and dry hydrogen chloride gas was introduced to saturation, and the reaction was stirred for about 20 hours, and the reaction solution was concentrated under reduced pressure, and then Ammonia water was added, and the oil layer was separated to obtain 111.2 g of DL-2-aminobutyric acid methyl ester with a yield of 95%.
[0046] In a 1 liter reaction flask, 105 g of methyl 2-aminobutyrate (0.9 mol) and 230 mL of methanol were added. Heat to 60°C, add 43 g of L-lactic acid, stir for 3 hours, filter, wash with an appropriate amount of methanol, and the filter cake is S-(+)-2-aminobutyric acid methyl ester L lactate solid. Add ethyl acetate to the solid of S-(+)-methyl 2-aminobutyrate L lactate, add an appropriate amount of solid sodium hydroxide, stir, separate the organic layer, dry, and recover the solvent to obtain S-(+) - Methyl 2-aminobutyrate 44 g. Yield 42...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com