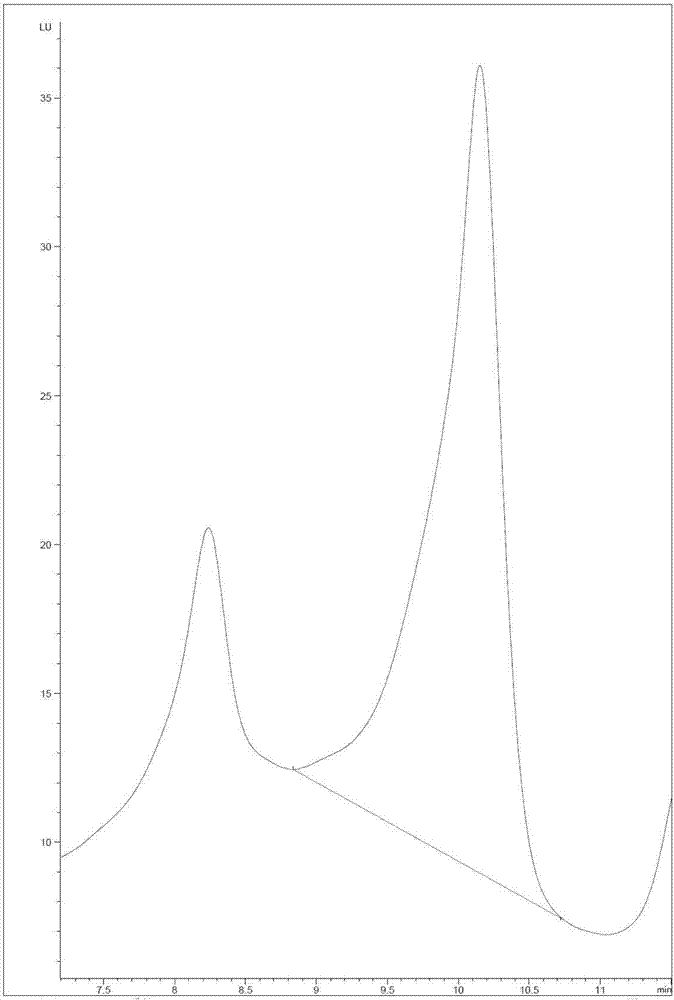
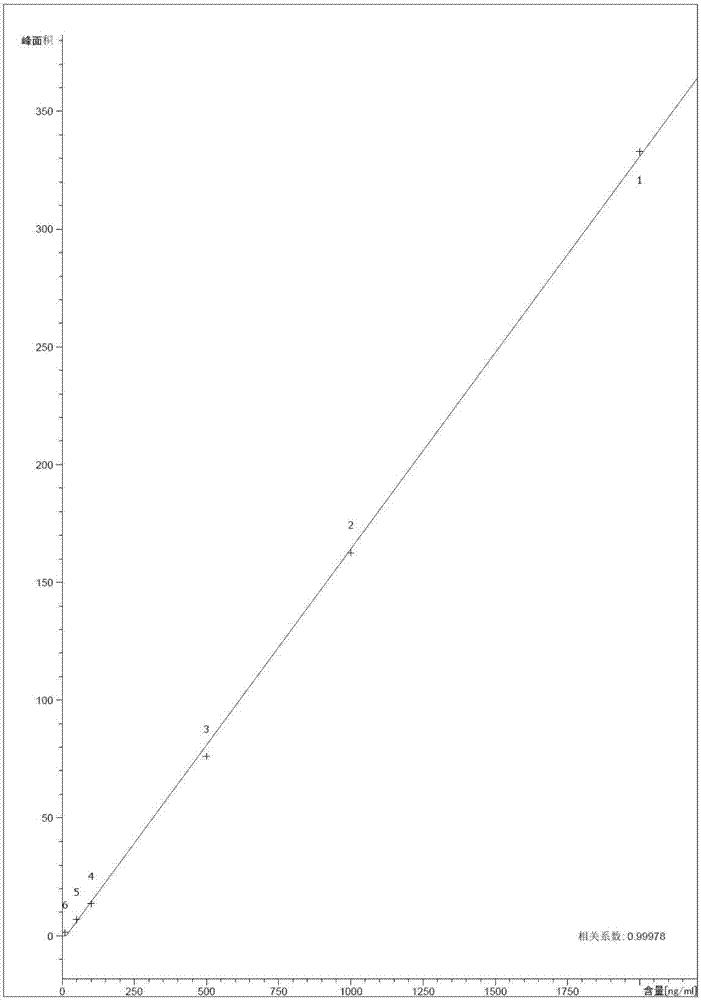
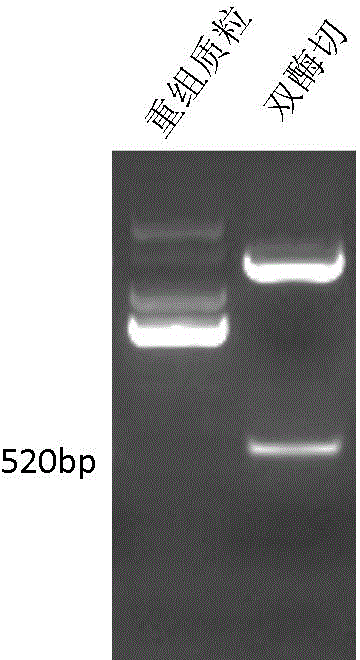
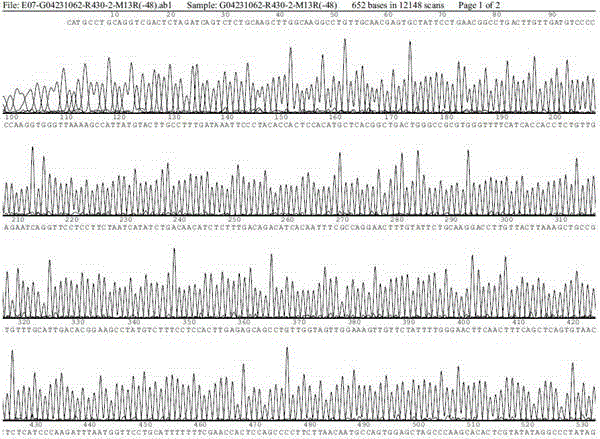
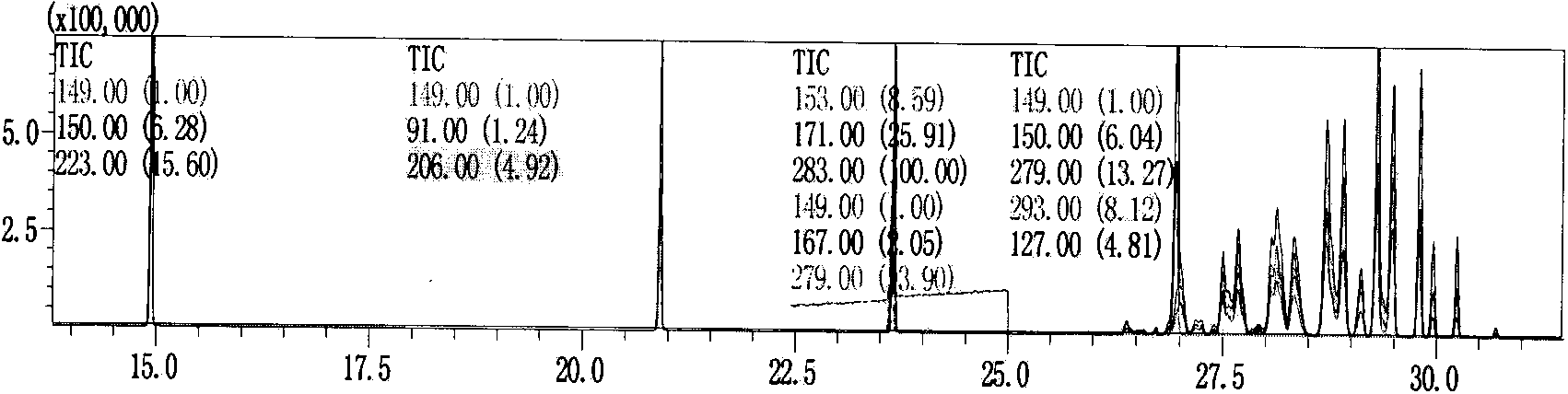
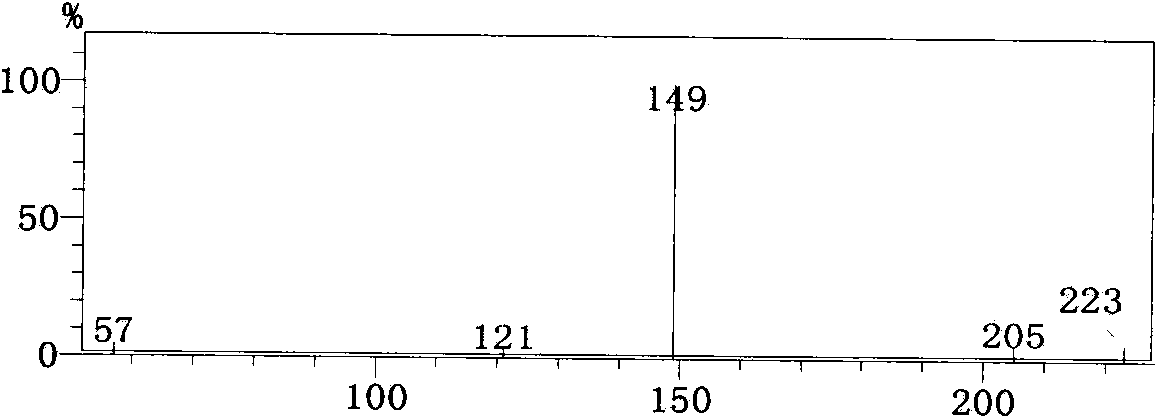
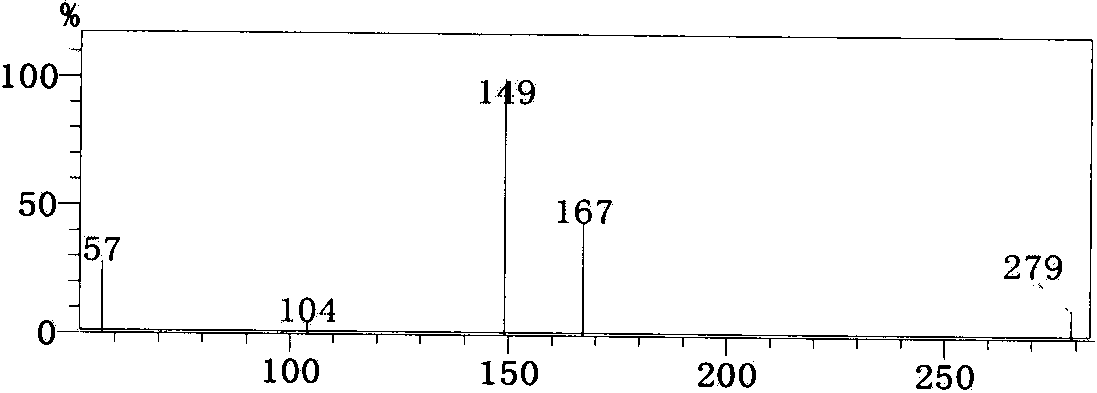
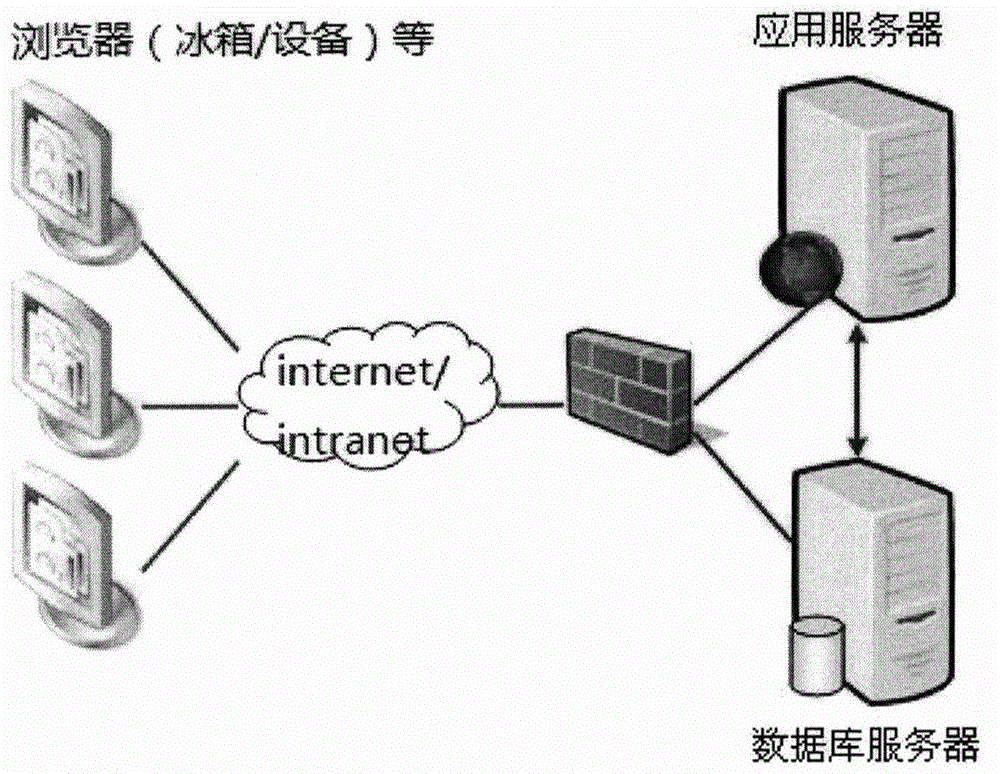
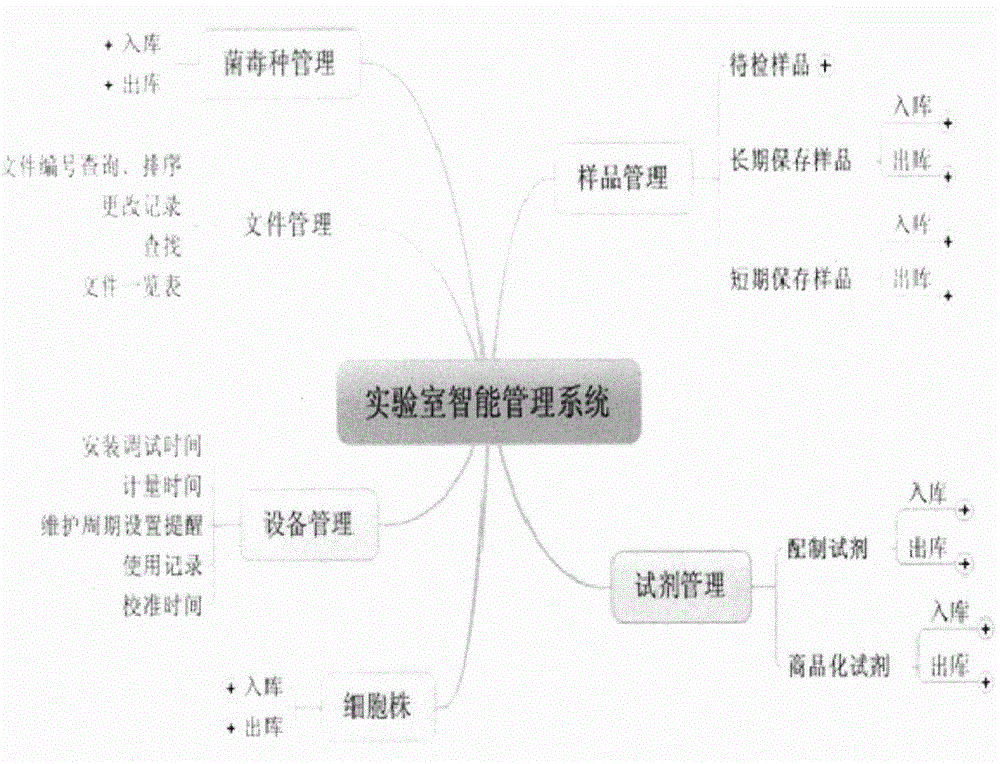
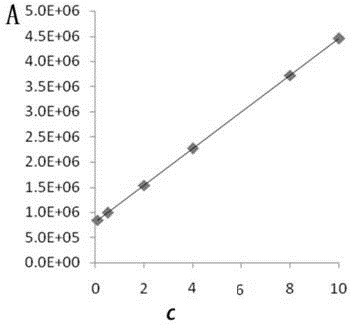
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Laboratory quality control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Laboratory quality control is designed to detect, reduce, and correct deficiencies in a laboratory's internal analytical process prior to the release of patient results, in order to improve the quality of the results reported by the laboratory. Quality control is a measure of precision, or how well the measurement system reproduces the same result over time and under varying operating conditions. Laboratory quality control material is usually run at the beginning of each shift, after an instrument is serviced, when reagent lots are changed, after calibration, and whenever patient results seem inappropriate. Quality control material should approximate the same matrix as patient specimens, taking into account properties such as viscosity, turbidity, composition, and color. It should be simple to use, with minimal vial to vial variability, because variability could be misinterpreted as systematic error in the method or instrument. It should be stable for long periods of time, and available in large enough quantities for a single batch to last at least one year. Liquid controls are more convenient than lyophilized controls because they do not have to be reconstituted minimizing pipetting error.
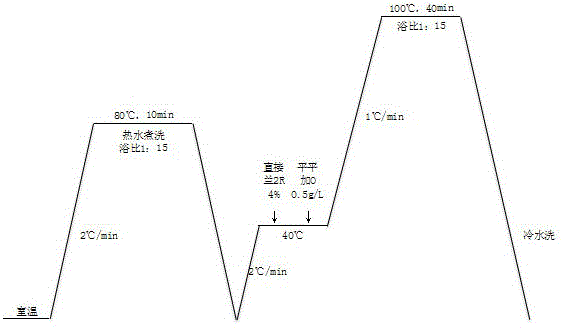
Capability verification sample for detection of textile color fastness to rubbing and preparation method thereof
InactiveCN104007061AEvenly dyedEasy to preparePreparing sample for investigationUsing mechanical meansColour fastnessRubbing
The invention discloses a capability verification sample for detection of textile color fastness to rubbing and a preparation method thereof. The capability verification sample is prepared by reactive dye or direct dye-based dying of pure cotton woven plain or twill bleached grey cloth. A test according to GB / T3920-2008 textile color fastness and color fastness to rubbing proves that color fastness to dry rubbing and color fastness to wet rubbing are in a range of 2-5 level, the existing daily detection range of color fastness to rubbing in the textile and clothing field is covered, and capability evaluation of different laboratory detection projects or laboratory quality control use is realized. The preparation method of the capability verification sample has simple processes, a high yield and high practicality. The capability verification sample can be stored and transported at a normal temperature and has uniformity and stability according with the CNAS-GL03 capability verification sample uniformity and stability evaluation guide requirements.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
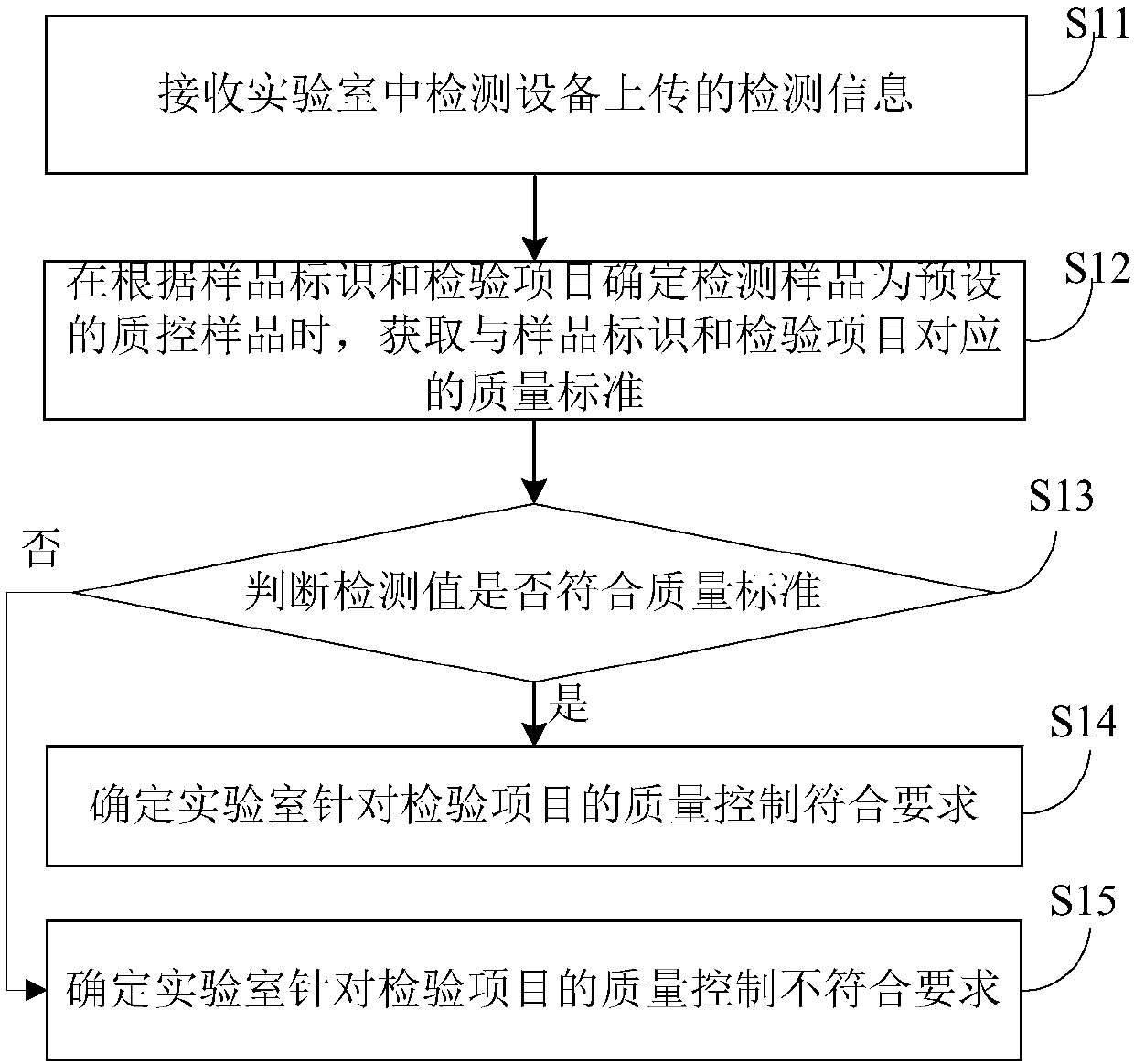
Laboratory quality control management method and system
InactiveCN107704986AQuality control scienceAccurate and efficient quality controlResourcesAutomatic judgementQuality control
The invention discloses a laboratory quality control management method and system. The method comprises the following steps of: receiving detection information uploaded by detection equipment in a laboratory, wherein the detection information comprises a sample identifier of a detection sample, an inspection item and a detection value corresponding to the inspection item; obtaining a quality standard corresponding to the sample identifier and the inspection item when determining the detection sample as a preset quality control sample according to the sample identifier and the inspection item;judging whether the detection value accords with the quality standard or not; and determining that quality control, aiming at the inspection item, in the laboratory accords with requirements if judging that the detection value accords with the quality standard. According to the method, the errors caused by human factors are avoided, the automatic judgement for detection values of quality control samples is realized, the quality control is more scientific, accurate and efficient, and the quality management, for the laboratory, of users is facilitated.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
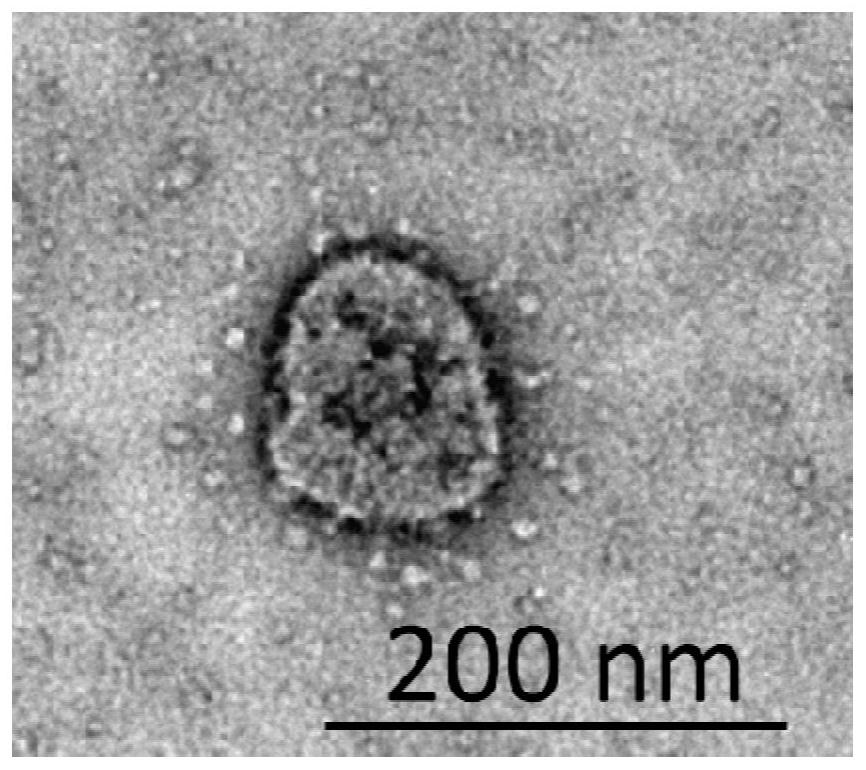
Novel coronavirus nucleic acid pseudovirus standard substance for detection and preparation method thereof
ActiveCN112359022AImprove securityImprove stabilitySsRNA viruses positive-senseMicrobiological testing/measurementRNA extractionFreeze-drying
The invention relates to a pseudovirus standard substance for nucleic acid detection of a novel coronavirus (2019-nCoV or called SARS-CoV-2) and a preparation method of the pseudovirus standard substance. Novel coronavirus nucleic acid is integrated into a lentiviral expression plasmid vector, then constructed lentiviral expression plasmids and packaging plasmids are jointly transfected into cells, and a large number of pseudoviral particles packaged with novel coronavirus RNA are obtained after cell expression. After plasmid DNA is removed through DNase I digestion and sucrose density gradient ultracentrifugation, freeze drying is performed to obtain a pseudovirus particle freeze-dried product. The pseudovirus standard substance has the partial RNA sequence packaged with the virus and also has a pseudovirus standard substance packaged with all the sequences, can completely simulate the whole process of virus RNA extraction and nucleic acid detection, and can be used for the verification evaluation and the laboratory quality control of the novel coronavirus nucleic acid qualitative and quantitative detection method. The obtained standard substance has the characteristics of high purity, good safety, accurate quantity value and the like, is good in stability, can be transported at normal temperature, and provides guarantee for quantity value traceability and transmission of thestandard substance.
Owner:NAT INST OF METROLOGY CHINA
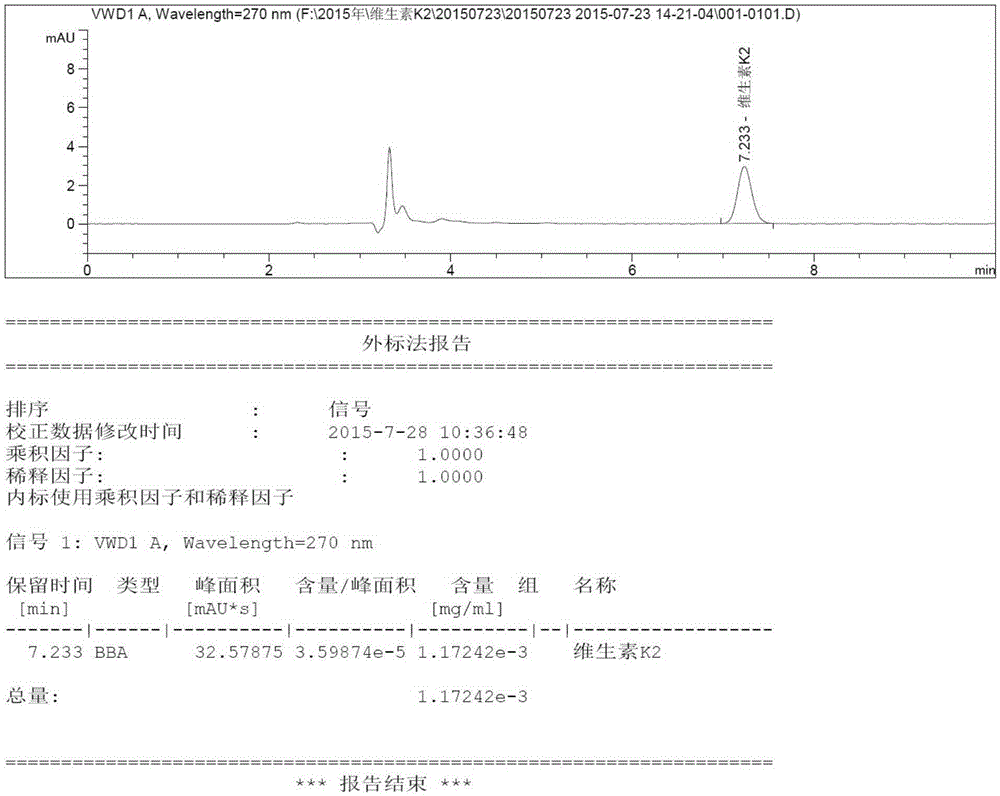
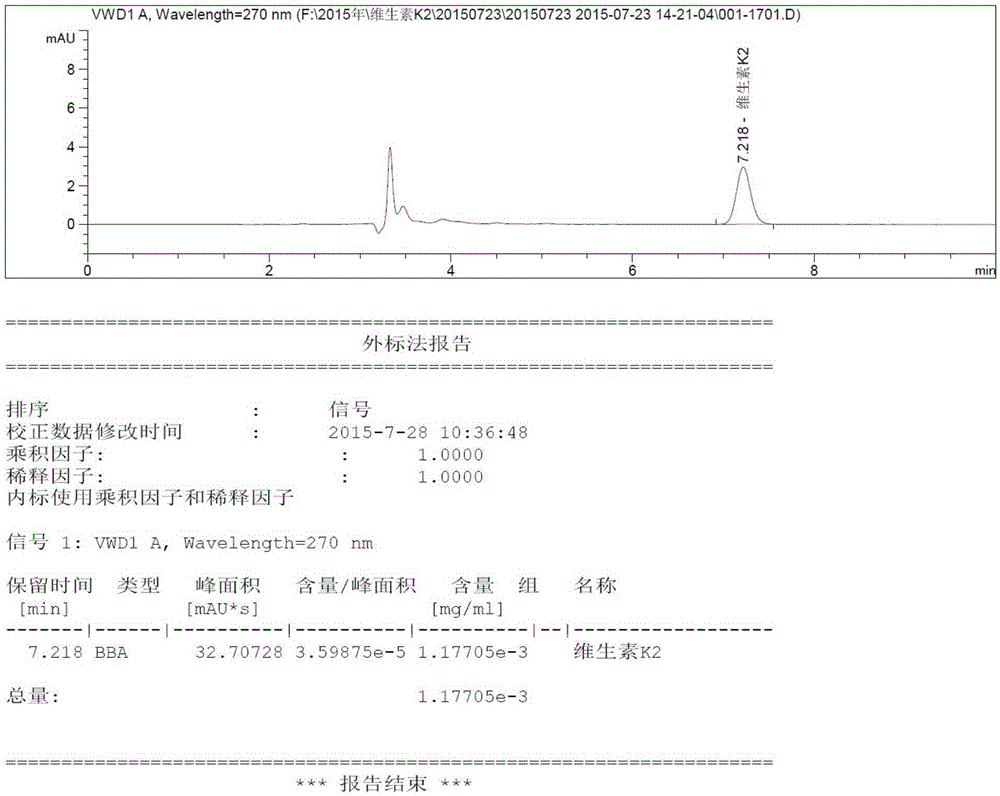
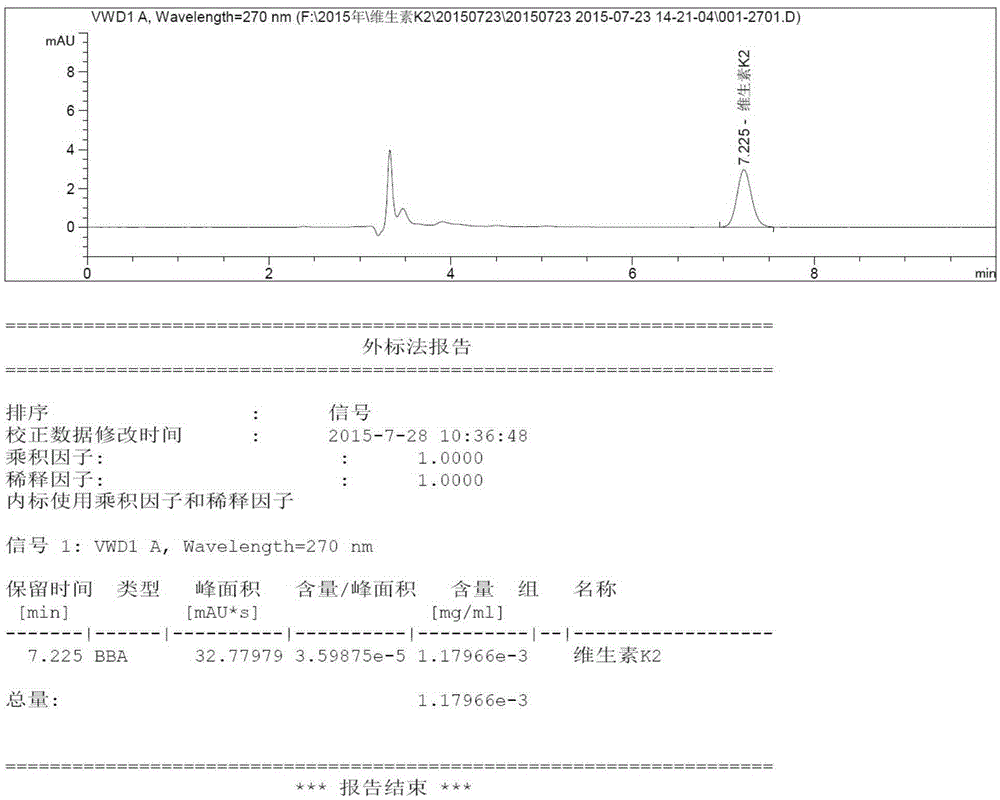
Method for extracting vitamin K2 from vitamin K2 embedding medium and method for detecting vitamin K2
InactiveCN105301148AThe content determination method is scientific and effectiveFor quality control purposesComponent separationVitamin K2Organic solvent
The invention relates to the field of analytical chemistry, in particular to a method for extracting vitamin K2 from a vitamin K2 embedding medium and a method for detecting the vitamin K2. According to the methods, the vitamin K2 embedding medium and acid are mixed, then subjected to ultraphonic processing, and then mixed with an organic solvent, the mixture is subjected to ultraphonic processing, and then the mixture is mixed with an organic solvent. The linearity, precision, repeatability, stability, blank, detection limit, quantitation limit and recovery experiments are carried out on the method for measuring the content of the vitamin K2, all the indexes meet the requirement of the GB / T27404-2008 laboratory quality control standard, it proves that the content measuring method is scientific and effective, and the quality control aim on the content of the vitamin K2 in the vitamin K2 compound preparation (embedding medium) can be achieved.
Owner:BY HEALTH CO LTD
Full automatic glass slide smear dyeing machine
PendingCN108106911AQuality assuranceEnsure consistencyPreparing sample for investigationComputer moduleEngineering
The invention discloses a full automatic glass slide smear dyeing machine, which comprises a slide and Tip head extraction module, a slide and Tip head frame, a sample frame, a label peeling module, afinished product slide cabin module, a slide dyeing module and a control module. The existing complicated morphologic sample pretreatment is converted into automatic equipment operation from manual operation; the labor cost of morphologic inspection is greatly reduced; the work efficiency of the morphologic inspection is improved; meanwhile, the specimen treatment quality is ensured. Meanwhile, the dropping liquid quantity is precisely controlled; the pellicle uniformity can be ensured. In addition, machine operation replaces manual operation of the gram stained slide; by aiming at the operation process, the quantitative control is performed; the dyeing effect and quality can be ensured; the dyeing effect deviation caused by different medical technological levels of different operation personnel is also avoided; the laboratory quality control management can be performed.
Owner:山东仕达思生物产业有限公司
Tetrodotoxin positive quality control sample as well as preparation method and application thereof
InactiveCN103983495AEasy to makeImprove stabilityPreparing sample for investigationPositive sampleTakifugu rubripes
The invention discloses a method for preparing a tetrodotoxin positive quality control sample. The method comprises the steps of selecting muscular tissues from fresh fugu rubripes, homogenizing, and measuring the content of tetrodotoxin in the muscular tissues to verify whether the muscular tissues contain the tetrodotoxin; if the muscular tissue is a positive sample, diluting the positive sample containing toxin with a negative sample and a dispersant, mixing uniformly, continuously measuring the content of the toxin in the sample, packaging the sample after the sample meets requirements, preserving at an appropriate temperature, and performing uniformity verification and stability analysis on the sample; if the muscular tissue is a negative sample, adding a tetrodotoxin standard solution into the negative samples, adding a dispersant, mixing uniformly, measuring the content of the tetrodotoxin in the sample, packaging the sample after the sample meets requirements, preserving at an appropriate temperature, and performing uniformity verification and stability analysis on the sample. The quality control sample is simple to operate, and has enough stability and uniformity and long preservation time, so that the quality control sample can be used for laboratory quality control.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
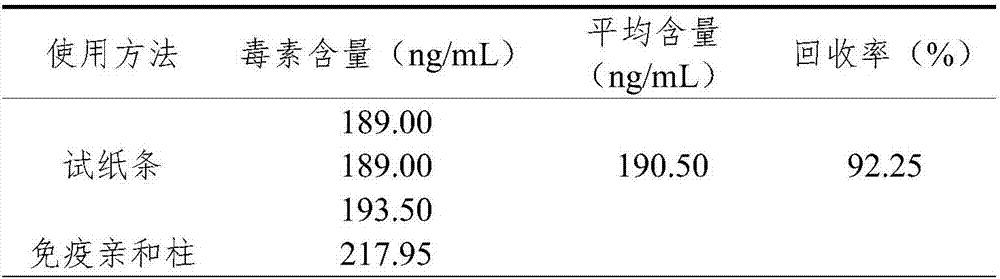
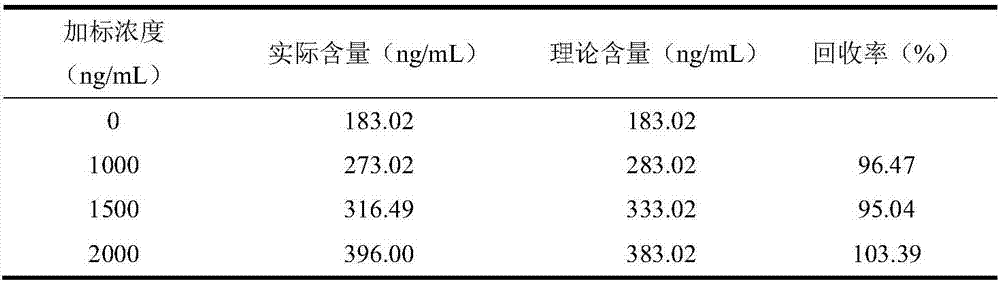
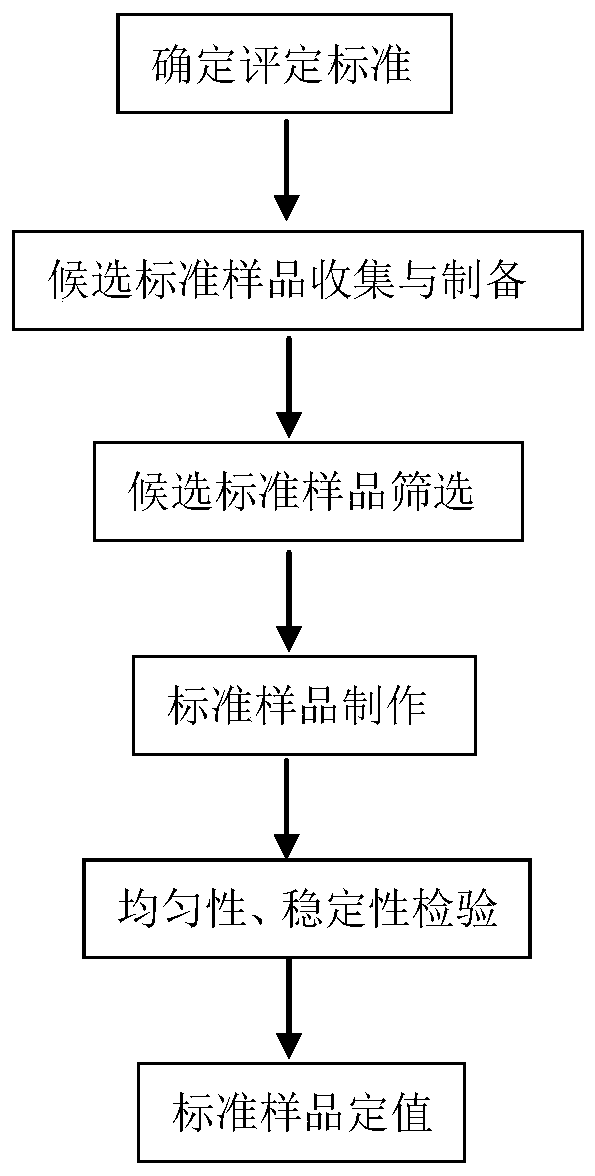
Method for performing reliability evaluation on result of quantitative detection item of detection system, and use
The invention provides a method for performing reliability evaluation on a result of a quantitative detection item of a detection system, and a use, and relates to the technical field of quality control of a medical laboratory. According to the method, actual experimental samples in the laboratory are used as evaluation samples, and there is no matrix effect compared with quality control. Representative samples are selected, that is, the experimental samples comprise an experimental sample in which a detection value of the quantitative detection item is less than a normal reference range, an experimental sample greater than the normal reference range, and an experimental sample within the normal reference range. In addition, the judging criteria of the method not only consider the requirements of quality objectives in the unique analysis of the detection item of the medical laboratory, but also consider the requirements of statistical linear regression. Therefore, the reliability of the result of the quantitative detection item of the detection system can be evaluated by the method in a simple and convenient, operable and efficient manner, and the method has an important practicalvalue on the quality control of the medical laboratory and the guarantee of the diagnosis and treatment of patients.
Owner:上海昆涞生物科技有限公司
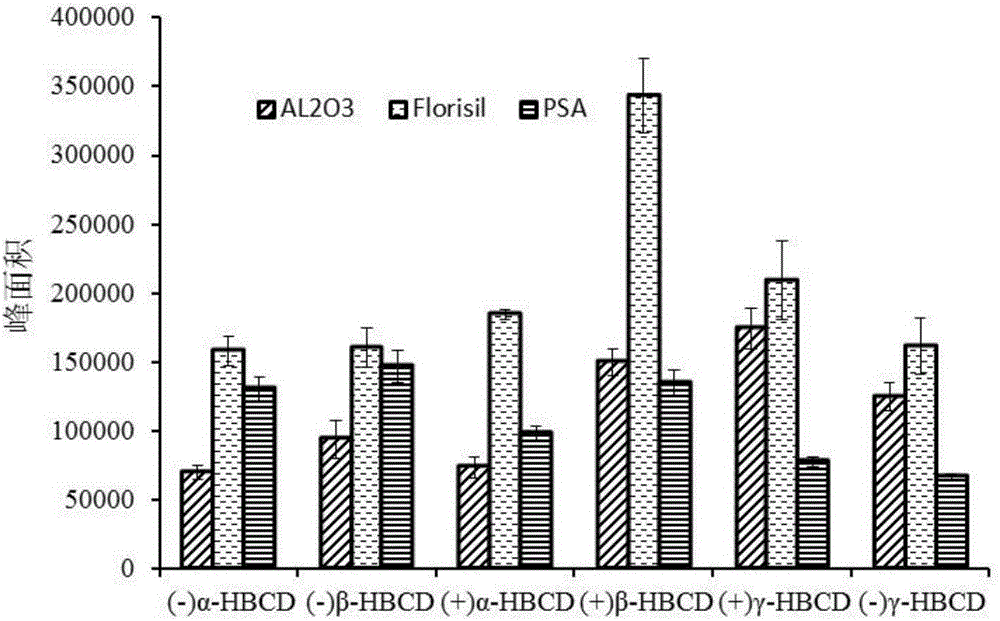
MSPD process based method for detecting hexabromocyclododecane chiral isomer in animal muscles
InactiveCN106770722AReduce consumptionSimple stepsComponent separationCorrelation coefficientFood safety
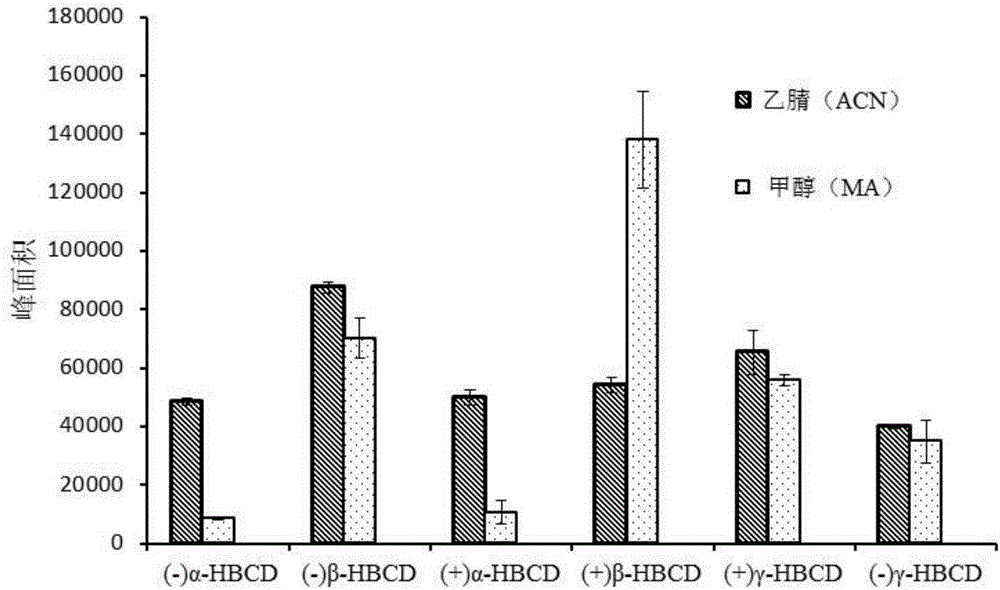
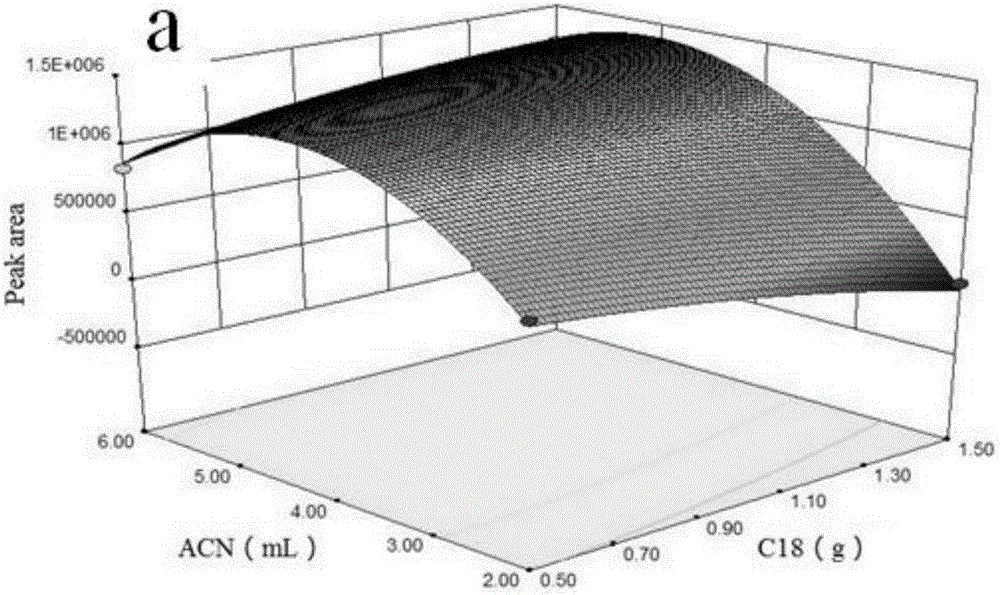
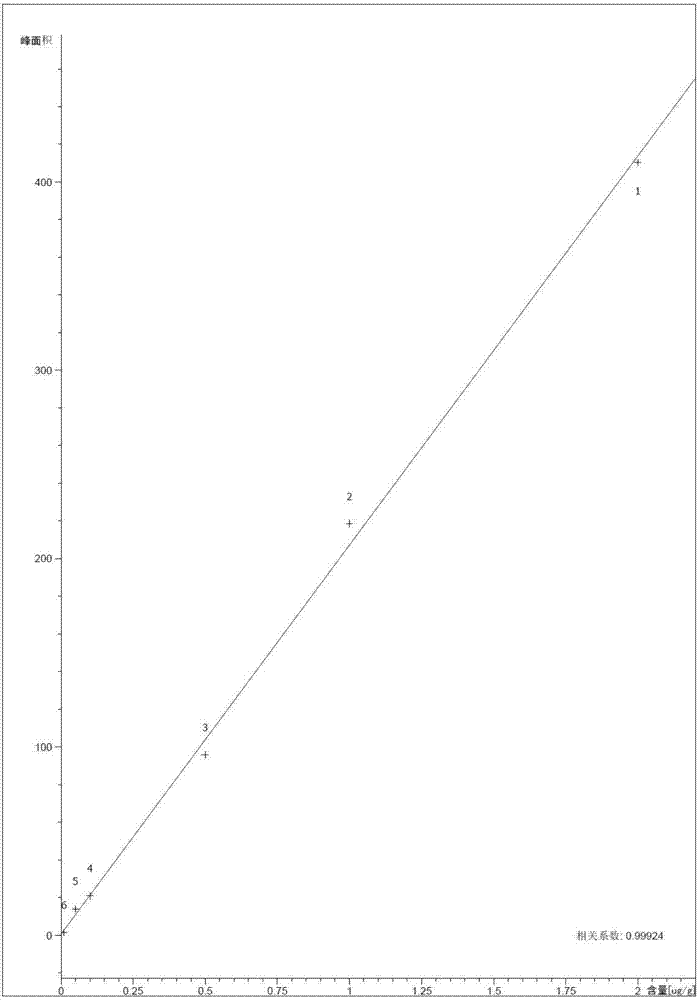
The invention discloses an MSPD process based method for detecting hexabromocyclododecane in animal muscles. Through a response surface method, the conditions of extracting HBCD from animal muscles in MSPD are optimized. Then the HBCD is detected by LC-MS / MS. The linear range of the detection method is 0.5 to 100 ng / g; the correlation coefficient is greater than 0.98; the LOD is 0.056 to 0.18 ng / g; the LOQs is 0.187 to 0.602 ng / g; the recovery rate is 84 to 117.3%; the precision RSD is 4.6 to 12.5%; and the national standards namely lab quality control specifications food physical and chemical testing (GB / T 27404-2008) are fully met. The pretreatment of the method is simple and rapid, the detection sensitivity is high, the results are accurate and reliable, and the method is environment-friendly and practical and has an important meaning for food safety, health of people, and social stability.
Owner:SHANDONG ANALYSIS & TEST CENT
Laboratory quality control and management system and method
The invention provides a laboratory quality control and management system and method. The laboratory quality control and management system comprises a mobile sampling process management and control system, a laboratory video monitoring system and a quality monitoring and dispatching system, wherein the mobile sampling process management and control system is installed in a hand-held mobile terminal of a user and used for receiving a sampling task, tracking and recording a moving trajectory in the sampling process and recording the sampling process by means of image shooting; the laboratory video monitoring system is installed in the laboratory and used for performing real-time monitoring on the analysis process conducted by an analyst on a sample in the laboratory; and the quality monitoring and dispatching system is used for performing storage and management on sampled sample data, sampling travel trajectories and sampled images provided by the mobile sampling process management and control system and laboratory monitoring video provided by the laboratory video monitoring system. The laboratory quality control and management system provided by the invention improves the authenticity of laboratory samples, the standardization of the sampling process and the reliability of the analysis process.
Owner:深圳博沃智慧科技有限公司
Detection method of citrinin toxin in red yeast rice
The invention belongs to the field of detection, and provides a detection method of citrinin toxin in red yeast rice. The method comprises the following steps: 1) accurately weighing a red yeast rice sample, grinding and sieving with a 20-mesh sieve; 2) uniformly mixing the red yeast rice sample with methanol, reacting and extracting a citrinin toxin solution, wherein the mass volume ratio of the red yeast rice sample to the methanol is 1g to 5mL; 3) detecting the citrinin toxin solution extracted in the step 1) with high performance liquid chromatography. The invention provides a method for extracting the citrinin toxin from the red yeast rice, which is easier and more convenient, thereby promoting the development of the red yeast rice and the red yeast rice industry. As proved by testing, the method can be used for rapidly extracting the citrinin toxin from the red yeast rice, has the advantages of high efficiency, low time consumption, low cost and high recovery rate, and conforms to the laboratory quality control specification-food physical and chemical testing standard; moreover, the extraction of the citrinin toxin from the red yeast rice and relevant products thereof is simplified greatly, and the method has great popularization and application values.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
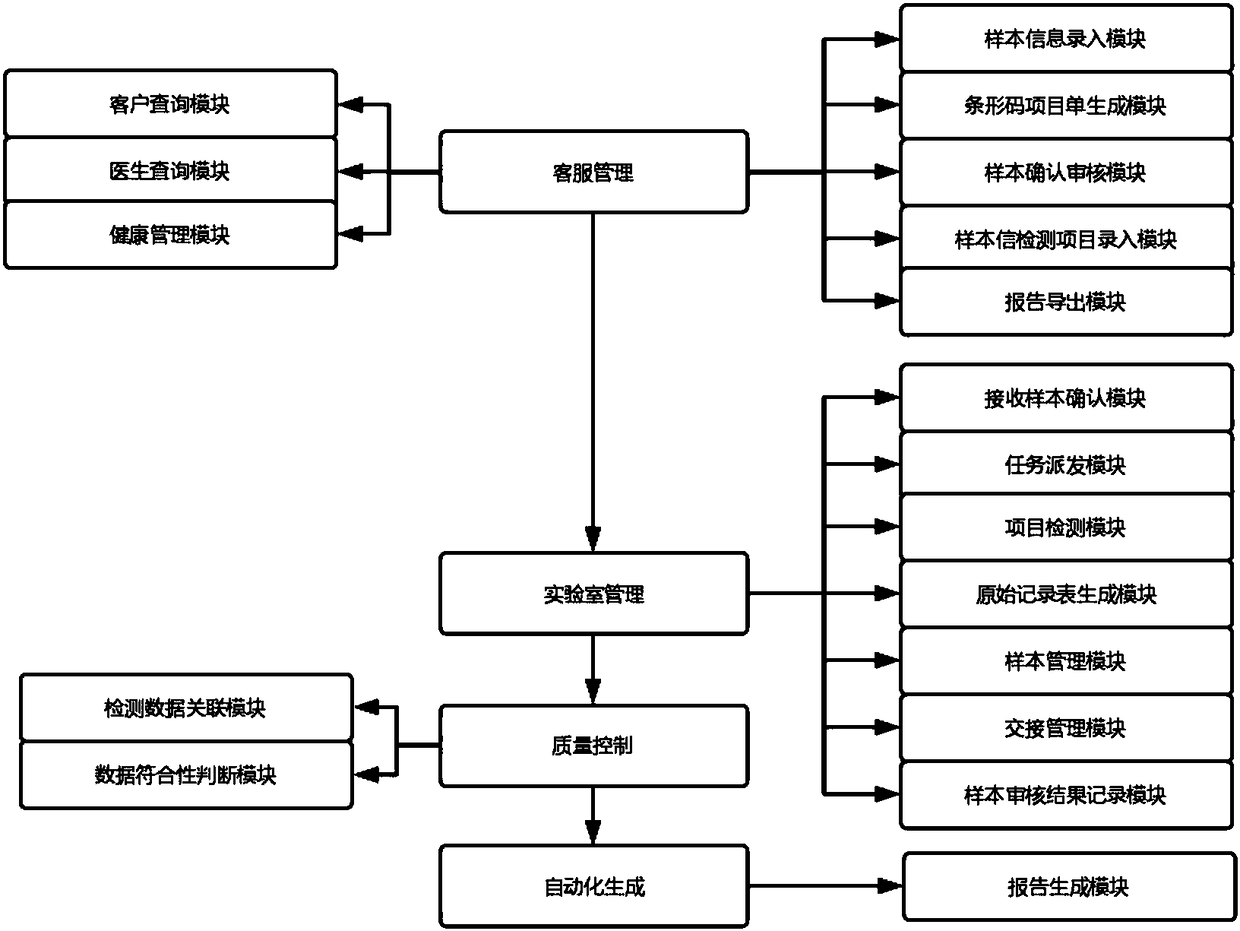
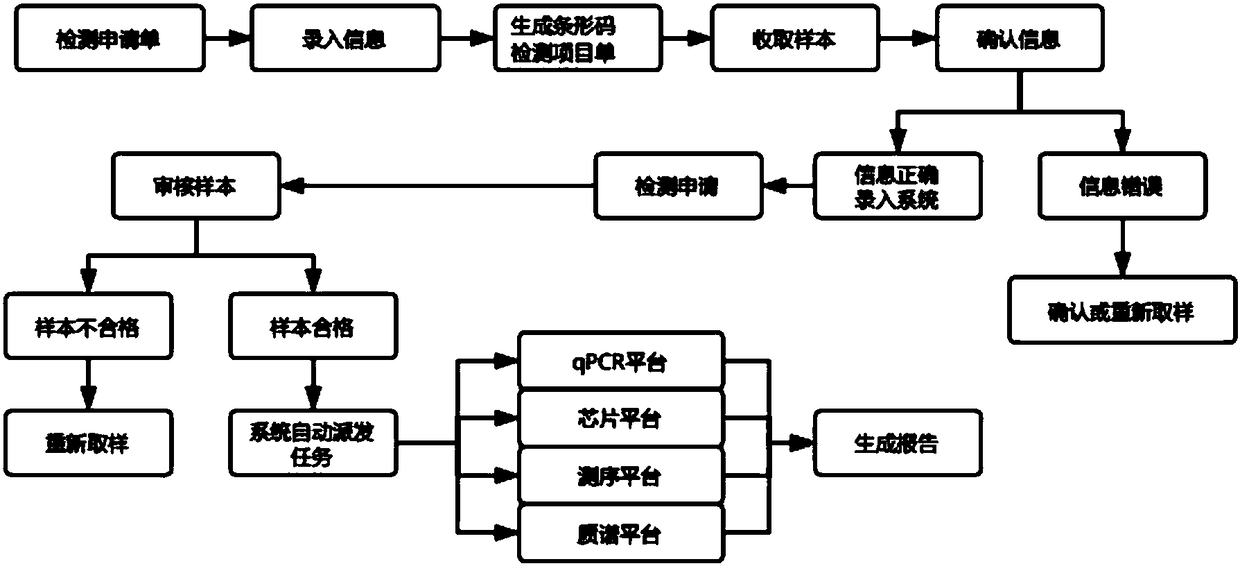
Customer service management, laboratory process, quality control and report automation generation system
ActiveCN108335724ASolve information dockingResolve auto-assignmentHealthcare resources and facilitiesLaboratory analysis dataThird partyProcess quality
The invention discloses a customer service management, laboratory process, quality control and report automation generation system, and relates to the technical field of laboratory management. The system comprises a customer service management module, a laboratory process management module, a laboratory quality control module and a report automation generation module. A popular B / S structure is adopted, and the functions of information matching between a third-party medical testing laboratory and a hospital, automatic distribution of each testing process quality detecting, and real-time generation of sample testing reports are effectively achieved; effective control of customer service management and testing process can be completely achieved by reasonable arrangement and related relationships among each module.
Owner:深圳市一道生物科技有限公司
Method for detecting citrinin toxin in monascus fermentation liquor
InactiveCN107192784AEasy extractionQuick extractionComponent separationLaboratory quality controlMonascus
The invention belongs to the field of detection and provides a method for detecting citrinin toxin in a monascus fermentation liquor. The method comprises the following steps: uniformly mixing the monascus fermentation liquor with methyl alcohol, wherein the mixing volume ratio is 1:(0.7-2); performing vibrating extraction; and performing high performance liquid chromatography. The method provided by the invention can quickly extract citrinin toxin from the monascus fermentation liquor, is high in efficiency, low in time consumption, low in cost, and high in recovery rate (<100 ng / mL, the recovery rate of 60-120%; 100-1,000 ng / mL, the recovery rate of 80-110%; and 1,000-100,000 ng / mL, the recovery rate of 90-110%), conforms to the laboratory quality control specifications-food physical and chemical testing standards, besides, greatly simplifies extraction of citrinin toxin from monascus and related products, and therefore, has an extremely high popularization and application value.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Xylo-oligosaccharide content detection method
ActiveCN105067718AEliminate distractionsAccurate determination of contentComponent separationCelluloseIsocratic elution
The invention relates to the field of analytical chemistry, in particular to a xylo-oligosaccharide content detection method. The detection method comprises steps as follows: a xylo-oligosaccharide test sample is taken and hydrolyzed, a hydrolyzed xylo-oligosaccharide test sample is obtained, HPLC (high performance liquid chromatography) is adopted for detection, the chromatographic condition of the HPLC is as follows: a saccharide column or an amino column is adopted, purified water or an acetonitrile aqueous solution is taken as a mobile phase for isocratic elution, and the xylo-oligosaccharide content is obtained according to an HPLC detection result. With the adoption of the detection method, interference of other functional saccharide cellulose can be eliminated, and the xylo-oligosaccharide content can be accurately detected; the xylo-oligosaccharide content detection method meets the requirement of the GB / T 27404-2008 Criterion on Quality Control of Laboratories-Chemical Testing of Food through linearity and precision tests and recovery rate tests, it proves that the detection method is scientific and effective, and the purpose of quality control on the xylo-oligosaccharide content detection can be achieved.
Owner:BY HEALTH CO LTD
Detection method of yeast glucan added into albumen powder
InactiveCN107976503AAccurate measurementThe detection method is scientific and effectiveComponent separationYeastLaboratory chemicals
The invention discloses a detection method of yeast glucan added into albumen powder. The detection method comprises steps of a) drawing a standard curve; b), extracting; c), carrying out hydrolytic treatment on a sample; d), detecting a to-be-detected sample by a chromatographic instrument; e), calculating to obtain a content of the yeast glucan in the to-be-detected sample, and the like. The interference of albumen, fat and a water-soluble saccharide can be eliminated according to the detection method provided by the invention; the content of the yeast glucan can be accurately measured; further, the detection method, through carrying out linear and precision tests and a recovery rate test on a content measurement method of the yeast glucan, all meets requirements of GB / T 27404-2008 Criterion on Quality Control of Laboratories-Chemical Testing of Food, and the detection method is proven to be scientific and effective, and can be used for realizing a quality control purpose to the content measurement of the yeast glucan.
Owner:BEIJING COMPETITOR SPORTS SCI & TECH
Crimean-Congo hemorrhagic fever virus nucleic acid molecule characteristic standard sample and its preparation method
InactiveCN104404168AImprove uniformityImprove stabilityMicrobiological testing/measurementMicroorganism based processesFreeze-dryingCrimean Congo hemorrhagic fever virus
The invention discloses a preparation method of a Crimean-Congo hemorrhagic fever virus nucleic acid molecule characteristic standard sample. The Crimean-Congo hemorrhagic fever virus nucleic acid molecule characteristic standard sample is prepared through sequence synthesis, vector cloning, target fragment and vector connection, plasmid transformation, recombinant plasmid extraction, freeze-drying and preservation, includes virus characteristic sequence information, and is suitable for analyzing the virus and the content value of the virus. The standard sample provided by the invention has the advantages of good uniformity, high stability, long-time storage and good purity. The preparation method has a simple process, can provide the standard sample for detection research and medical research of Crimean-Congo hemorrhagic fever virus to realize the comparison of different laboratory results in order to ensure the laboratory quality control, and also provides the standard sample for the rapid and accurate detection of Crimean-Congo hemorrhagic fever virus by inspection and quarantine institutions, and import and export trade enterprises.
Owner:薛芳
Laboratory quality control technology for phthalate detection in wine and application thereof
InactiveCN104280465AGuaranteed repeatabilityGuaranteed stabilityComponent separationGas phaseRetention time
The invention discloses a laboratory quality control technology for phthalate detection in wine and application thereof. The detection technology is as follows: extracting phthalate in a sample with n-hexane, taking a supernatant for dehydration, using d4-di (2-ethyl) hexyl phthalate (d4-DEHP) as an internal standard for detecting by gas chromatography-mass spectrometry. By using feature selected ion monitoring scan mode (SIM), chromatographic retention time and debris abundance ratio are taken for determination on the nature and an internal standard method is taken for determination on the quantify. The method provided by the invention is simple, good in stability, high in sensitivity and convenient to use, and can realize the rapid on-site detection of the phthalate.
Owner:王传现 +2
Key quality control factor management system of animal quarantine laboratory
InactiveCN105528665ATimely borrowingImplement data entrySensing record carriersResourcesOriginal dataThe Internet
The invention provides a key quality control factor management system of an animal quarantine laboratory, and aims at overcoming the problems that laboratory management is low in efficiency and causes resource waste traditionally. An RFID technology is used to provide each person or object with a unique ID, key factors including samples, reagents, cell strains, files and devices related to the laboratory are covered comprehensively, a windows operating system, B / S configuration and a database are combined, a life period that can be monitored is entered after that original data is input manually, the working efficiency of operators is greatly improved, use of consumable articles is reduced, and cross-regional purchasing and sharing of laboratory systems are realized via 3G and the Internet.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT
Method for detecting 20 volatile components in tea
PendingCN111879867AAchieve separationRealize simultaneous qualitative and quantitative detectionComponent separationLinear correlationChemical compound
The invention discloses a method for detecting 20 volatile components in tea, which comprises the following steps of: establishing a steam distillation extraction method of the volatile components inthe tea and an accurate qualitative and quantitative analysis method of the 20 components by taking a standard substance as a reference; by adopting the method provided by the invention, the separation degree of chromatographic peaks of a target compound and a standard substance is relatively good, each component in a concentration range of 0.1 mu g / mL to 10mu g / mL has relatively good linear correlation, and the related requirements of GB / T 274042008 Food Physical and Chemical Detection Quantitative Requirements of Laboratory Quality Control Standards are met. Reference is provided for analysis of chemical components and contents of volatile components of tea, favorable conditions are provided for deep analysis of aroma threshold values and smell characteristic contribution degrees of thevolatile components in the tea, and the processing technology can be conveniently and accurately regulated and controlled to improve the quality of the tea.
Owner:PUER COMPREHENSIVE TECHN TESTING CENT
Method for extracting and detecting citrinin toxin in red preserved bean curd
The invention belongs to the field of detection and provides a method for extracting and detecting citrinin toxin in red preserved bean curd. The method comprises the following steps: 1) uniformly mixing a red preserved bean curd sample and methanol; reacting and extracting a citrinin toxin solution, wherein the mass / volume ratio of the red preserved bean curd sample to the methanol is 1g to 5mL; 2) detecting the extracted citrinin toxin solution by utilizing a high performance liquid chromatography. By utilizing a physicochemical property that citrinin is easily dissolved into organic solvents including acetonitrile, the methanol, ethyl acetate, acetone and the like, the invention provides a more simpler method for extracting and detecting the citrinin toxin in the red preserved bean curd and the development of monascus and a monascus industry is easily propelled. By adopting the method provided by the invention, the citrinin toxin in the red preserved bean curd can be rapidly extracted and the method has high efficiency, less consumed time, low cost and high recycling rate; laboratory quality control specifications-food physicochemical detection standards are met; furthermore, the extraction of the citrinin toxin in the monascus and related products thereof is extremely simple and convenient to realize by utilizing the method and the method has extremely high popularization and application value.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Manufacture and application methods of hepatitis B surface antigen serum laboratory quality control material
InactiveCN106198988AQuality improvementImprove accuracyMaterial analysisMedicineContinual improvement process
The invention discloses manufacture and application methods of a hepatitis B surface antigen serum laboratory quality control material. The manufacture method comprises five steps as follows: acquisition and screening of raw materials, diluted serum manufacturing, preservation and monitoring of a standby quality control material, quality control, continuous improvement, etc. The application method comprises three steps as follows: storage, re-dissolution and application. The manufacture and application methods of the hepatitis B surface antigen serum laboratory quality control material are complete and clear. Due to the usage of a quality control chart of multiple impact factors, accuracy is high and control range is wide. By adoption of 31S quality control limit which is very sensitive to the system problem of unreasonable fundamental technological processes caused by insufficient technical capacity in the production process, the method is helpful for guaranteeing accuracy of the technological direction and determining good technological foundation and optimizing production technology.
Owner:CHANGSHA KINGMED MEDICAL DIAGNOSTICS INST
Method for extracting citrinin toxin from red starter wine
InactiveCN107238525AEasy extractionQuick extractionComponent separationPreparing sample for investigationCitrininQuality control
The invention discloses a method for extracting citrinin toxin from red starter wine. The method disclosed by the invention comprises the following steps: uniformly mixing the red starter wine with methanol, and reacting, so that a product is obtained, namely the citrinin toxin is obtained. Experiments prove that the method disclosed by the invention has the advantages that the citrinin toxin can be rapidly extracted from the red starter wine, with high efficiency, short consumed time, low cost and high recovery ratio, so that laboratory quality control specification-food physical and chemical detection standard (GB / T 27404) can be met; besides, the method disclosed by the invention also greatly simplifies extraction of the citrinin toxin from red starter and related products and has extremely high popularization and application values.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Cholesterol content detection method
ActiveCN106198798AEasy to handleReduce processing timeComponent separationLaboratory quality controlChemistry
The invention relates to the field of quality control technology, especially to a cholesterol content detection method. The detection method comprises the following steps: a sample to be measured is dissolved in an organic solvent to obtain a test solution, and content of cholesterol in the test solution is detected by a gas chromatography-mass spectrum technology, wherein the organic solvent is one selected from isooctane, ether and hexane or a mixture of more than two selected from isooctane, ether and hexane. By the detection method, detection time can be greatly shortened, and detection accuracy can be raised. through the cholesterol content detection method, linearity, precision, repeatability, stability, blank, detection limit, quantification limit and recovery rate tests all meet requirements of GB / T27404-2008 ''laboratory quality control specification''. It proves that the method is scientific and effective and can be adopted for achieving the purpose of quality control of cholesterol content in animal and plant samples.
Owner:BY HEALTH CO LTD
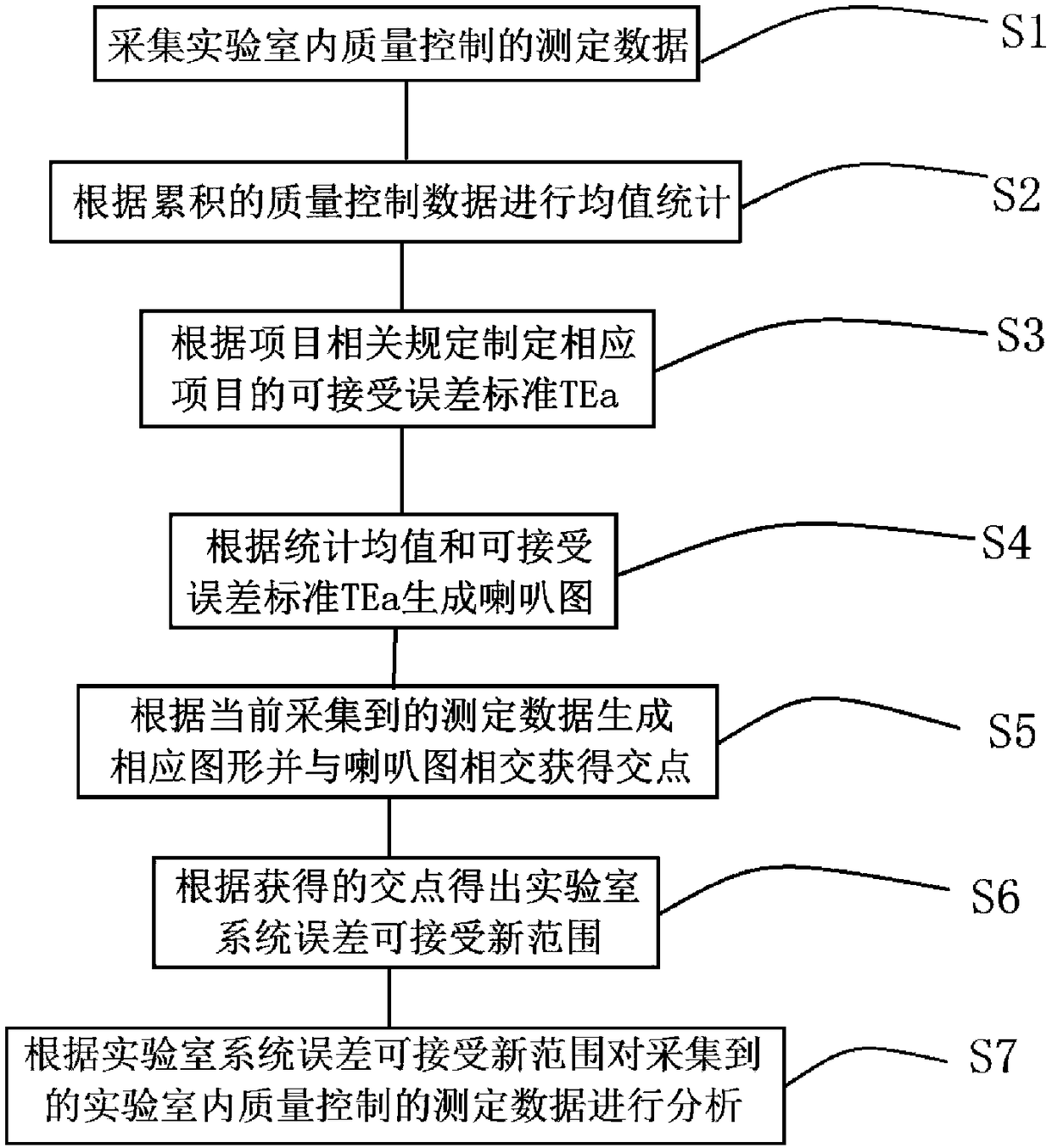
Laboratory quality control data management method
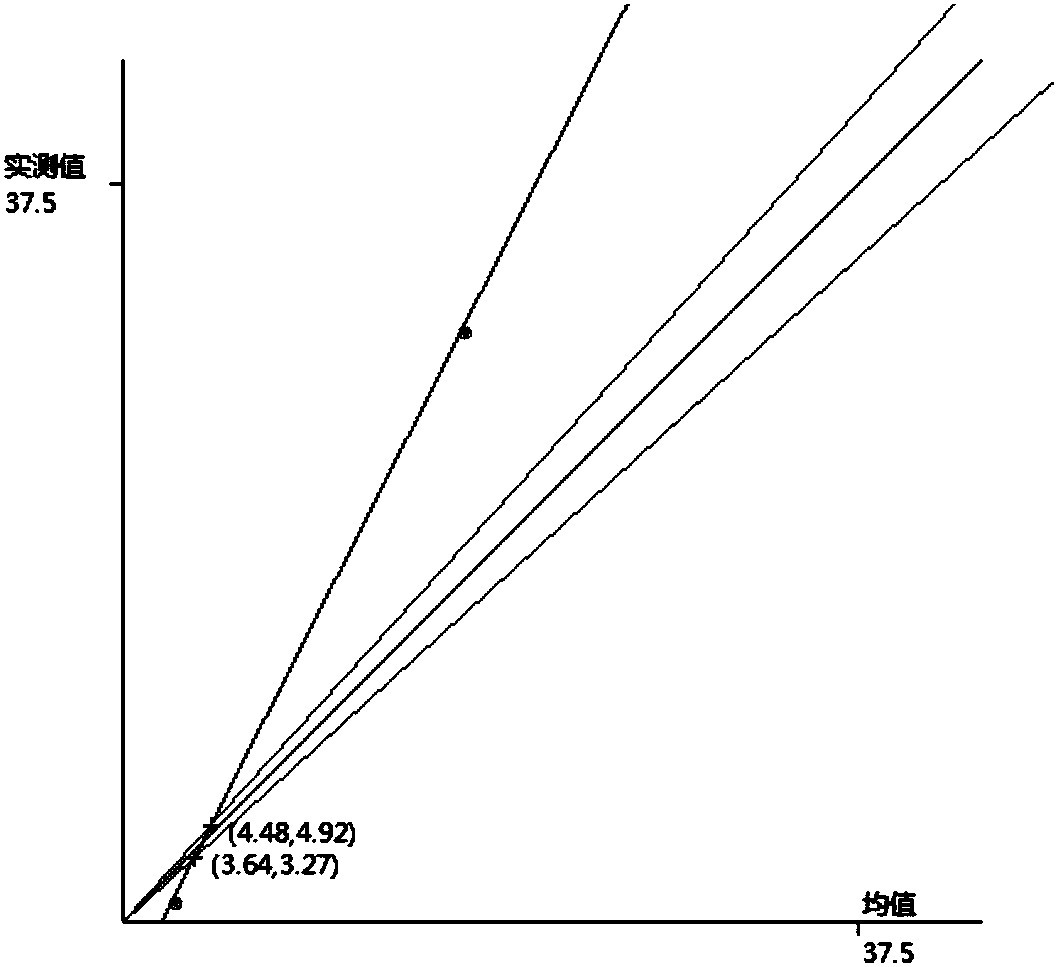
The invention discloses a laboratory quality control data management method comprising the following steps: collecting measurement data of quality control in a laboratory; making statistics of the mean of the accumulated quality control data; setting the allowable error standard TE of a corresponding item according to the relevant regulations of the item; generating a horn graph according to the statistical mean and the acceptable error standard TE; generating a corresponding graph according to currently collected measurement data, and making the graph intersect with the horn graph to obtain an intersection point; getting a new allowable range of system error of the laboratory according to the intersection point; and analyzing the collected measurement data of quality control in thelaboratory according to the new allowable range of system error of the laboratory. An analysis method effectively using the existing data is provided. Under the condition that there is system error, the existing data is analyzed to obtain a narrower acceptable temporary range of total error. The detection result can meet the total error requirement, and is accepted. There is no need to reject allsample results. The resources are effectively utilized.
Owner:上海昆涞生物科技有限公司
Method for quickly detecting zearalenone toxin in vegetable oil
InactiveCN107271431AEasy to detectQuick checkComponent separationAnalysis by subjecting material to chemical reactionOrganic solventAlcohol
The invention discloses a method for quickly detecting zearalenone toxin in vegetable oil. The method comprises the steps of uniformly mixing the vegetable oil and a methanol solution, centrifuging or carrying out settling separation after reaction, getting a supernatant, and utilizing a zearalenone toxin test strip for detecting. According to the detection method provided by the invention, the physicochemical property that zearalenone is easy to dissolve in organic solvents such as acetonitrile, methyl alcohol, ethyl alcohol, ethyl acetate and acetone is utilized, so that the method for simply and conveniently detecting zearalenone toxin in the vegetable oil is invented, and the development of oil and vegetable oil industries is promoted. The tests show that the method provided by the invention can be used for quickly detecting the zearalenone toxin in the vegetable oil, is high in efficiency, less in consuming time, low in cost and high in recovery rate, and meets the laboratory quality control standard-food physical and chemical detection standard.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Determination method of content of inorganic phosphorus in sodium phytate
InactiveCN105352783AReasonable methodSimple and fast operationPreparing sample for investigationRelative standard deviationVanadate
The invention discloses a determination method of the content of inorganic phosphorus in sodium phytate. The determination method comprises the following steps: firstly, adding a ferric trichloride solution into a sodium phytate sample solution to form sediment [C6H6(OH)H3(PO4)6]3Fe; removing base material influences of the sodium phytate by using a chelating effect of phytic acid and metal ions; centrifuging at a high speed to remove the sediment and adding a vanadate molybdate solution to generate a yellow phosphorus-vanadium molybdate coordination compound; determining a light absorption value at 420nm by using a spectrophotometer; calculating the content of the inorganic phosphorus in the sample according to a standard curve. According to the determination method provided by the invention, a base material effect is removed by using ferric trichloride and then a vanadium-molybdenum-yellow method is used for determining the content of the inorganic phosphorus; the method is reasonable and is simple and convenient to operate; the problems that the inorganic phosphorus in the sodium phytate is detected and has no recycling, and the base material interference is eliminated are solved; when the adding concentration is 0.02 percent to 0.08 percent, the recycling rate ranges from 99.0 percent to 102.48 percent, and the relative standard deviation is smaller than 1.16 percent; the technological indexes meet the requirements of GB / T 27404-2008 Food Physicochemical Detection of Laboratory Quality Control Standards.
Owner:INSPECTION & QUARANTINE COMPREHENSIVE TECH CENT JIANGXI ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Corn imperfect grain standard sample and preparation method thereof
ActiveCN110926905AHigh transparencyEasy to observe fine structurePreparing sample for investigationAgricultural engineeringMildew
The invention discloses a corn imperfect grain standard sample and a preparation method thereof. The corn imperfect grain standard sample is prepared by embedding insect-eroded grains, scab grains, broken grains, germinated grains, mildew grains and heat-damaged grains by organic resin. According to the invention, an organic resin embedding technology is adopted to manufacture a corn imperfect grain standard sample, the corn imperfect grain standard sample has excellent properties such as flame retardance, aging resistance, non-toxicity, oxidation resistance, heat resistance, insulation and the like, and provides a reliable and scientific material basis for quality control activities in the field of inspection; and laboratory quality control is facilitated, improvement of laboratory quality management level is facilitated, and risks existing in accuracy of laboratory inspection results are reduced.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
A method for detecting the content of organic synthetic pigments
ActiveCN107305203BProve that the content determination method is scientific and effectiveFor quality control purposesComponent separationOrganic synthesisGradient elution
The invention relates to the technical field of quality control and especially relates to a detection method for organic artificial color content. The detection method comprises the following steps: mixing a sample with alkali liquor and regulating pH value to 7, thereby acquiring a tested liquor, and then adopting high performance liquid chromatography for detecting the tested liquor and acquiring the organic artificial color content according to a content detection result of a standard substance. The chromatographic conditions of the high performance liquid chromatography are as follows: flowing phases: ammonium acetate is served as a flowing phase A and acetonitrile is served as a flowing phase B; elution process: gradient elution; chromatographic column: C18 chromatographic column; enticed red and / or sodium indigotin-disulfonate is served as the organic artificial color. The linearization, precision, repeatability, stability, blank, detection limit, quantitation limit and recovery rate of detection method provided by the invention all meet the requirement of GB / T27404-2008 'Laboratory Quality Control Specification'; the content detection method is proved as scientific and effective; the purpose of quality control for the content of enticed red and / or sodium indigotin-disulfonate in a tablet can be achieved.
Owner:BY HEALTH CO LTD
A kind of tetrodotoxin positive quality control sample and its preparation method and application
InactiveCN103983495BEasy to makeImprove stabilityPreparing sample for investigationMuscle tissueBiochemical engineering
The invention discloses a method for preparing a tetrodotoxin positive quality control sample. The method comprises the steps of selecting muscular tissues from fresh fugu rubripes, homogenizing, and measuring the content of tetrodotoxin in the muscular tissues to verify whether the muscular tissues contain the tetrodotoxin; if the muscular tissue is a positive sample, diluting the positive sample containing toxin with a negative sample and a dispersant, mixing uniformly, continuously measuring the content of the toxin in the sample, packaging the sample after the sample meets requirements, preserving at an appropriate temperature, and performing uniformity verification and stability analysis on the sample; if the muscular tissue is a negative sample, adding a tetrodotoxin standard solution into the negative samples, adding a dispersant, mixing uniformly, measuring the content of the tetrodotoxin in the sample, packaging the sample after the sample meets requirements, preserving at an appropriate temperature, and performing uniformity verification and stability analysis on the sample. The quality control sample is simple to operate, and has enough stability and uniformity and long preservation time, so that the quality control sample can be used for laboratory quality control.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Corn imperfect kernel standard sample and its preparation method
ActiveCN110926905BHigh transparencyEasy to observe fine structurePreparing sample for investigationLaboratory Test ResultBiochemical engineering
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
A method for detecting cholesterol content
ActiveCN106198798BEasy to handleReduce processing timeComponent separationOrganic solventCholesterol
The invention relates to the field of quality control technology, especially to a cholesterol content detection method. The detection method comprises the following steps: a sample to be measured is dissolved in an organic solvent to obtain a test solution, and content of cholesterol in the test solution is detected by a gas chromatography-mass spectrum technology, wherein the organic solvent is one selected from isooctane, ether and hexane or a mixture of more than two selected from isooctane, ether and hexane. By the detection method, detection time can be greatly shortened, and detection accuracy can be raised. through the cholesterol content detection method, linearity, precision, repeatability, stability, blank, detection limit, quantification limit and recovery rate tests all meet requirements of GB / T27404-2008 ''laboratory quality control specification''. It proves that the method is scientific and effective and can be adopted for achieving the purpose of quality control of cholesterol content in animal and plant samples.
Owner:BY HEALTH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com