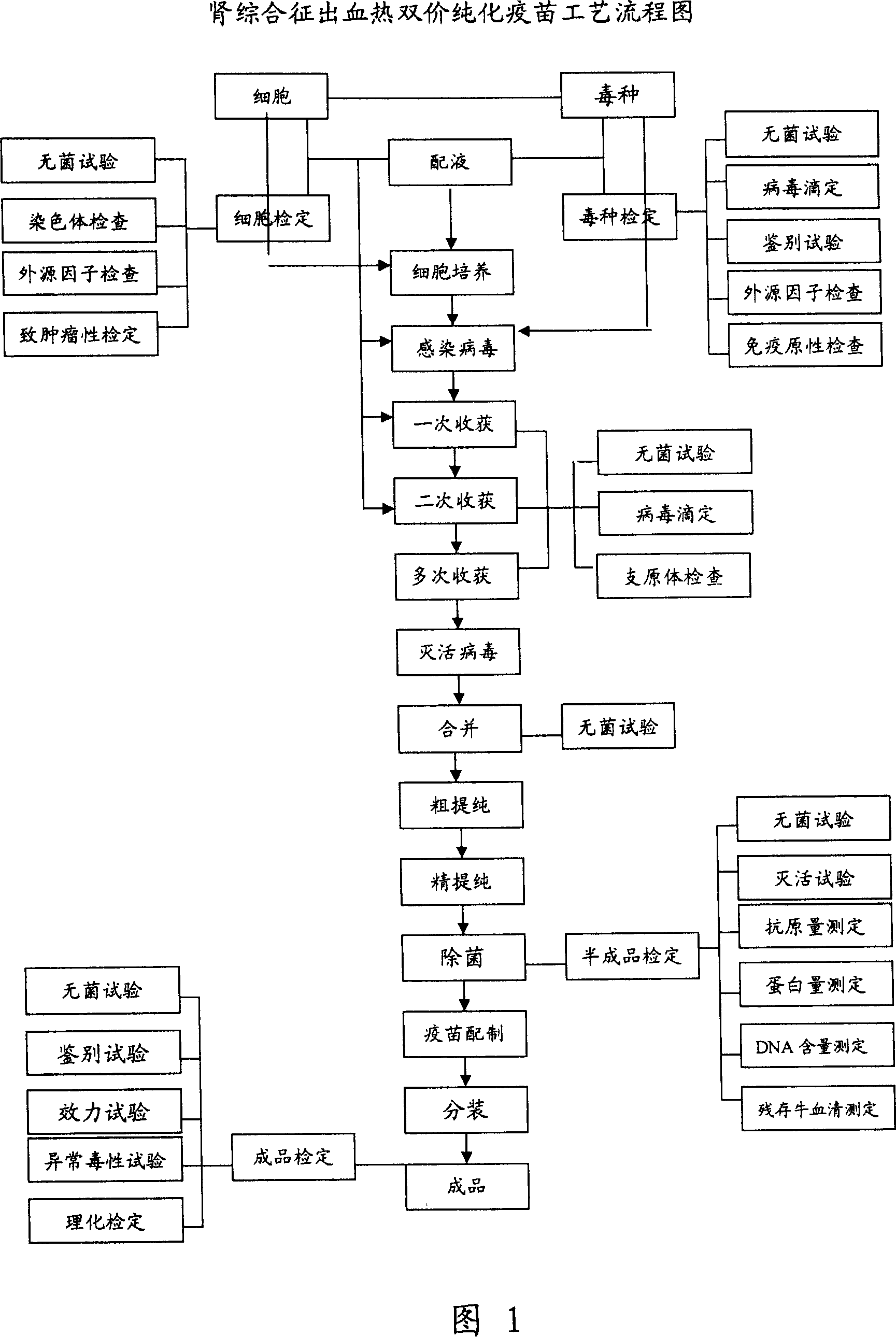
Kidney syndrome hemorrhagic fever Vero cell bivalent purified vaccine and industrialized producing process thereof
A technology for renal syndrome and hemorrhagic fever, which can be applied to pharmaceutical formulations, viral antigen components, antibody medical components, etc., and can solve the problems of high vaccine costs and low harvest times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Vero cell culture
[0088] Take a few bottles (3L spinner bottles) of Vero cells grown into a monolayer (from the China Institute for the Control of Pharmaceutical and Biological Products), discard the growth solution, add trypsin solution with a pH of 8.0 and a concentration of 0.1%, and place it on a spinner at room temperature. Perform a spin digest. When the digestive line appears on the cell surface with the naked eye, discard the trypsin solution, add an appropriate amount of 199 culture solution (growth solution) containing 8-10% calf serum, shake the solution gently to wash the cells off the bottle wall, and suspend the cells in in the growth fluid. After shaking well, divide it into 3 liter spinner bottles at a ratio of 1 to 3, add growth solution to each bottle to 300ml, and place it at 37°C for rotary culture. After three days, the cells grow into a monolayer.
[0089] Separate preparation of PS-6(C-3) type I vaccine stock solution and L99(C-2) type II vacc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com