Method for rapidly preparing Zika-virus specific full human monoclonal antibodies and application
A monoclonal antibody, Zika virus technology, applied in the field of medicine and biology, can solve problems such as unfavorable large-scale promotion and application, loss, etc., and achieve the effects of simple and fast method, improved efficiency and time saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. A method for rapidly preparing Zika virus-specific fully human monoclonal antibodies
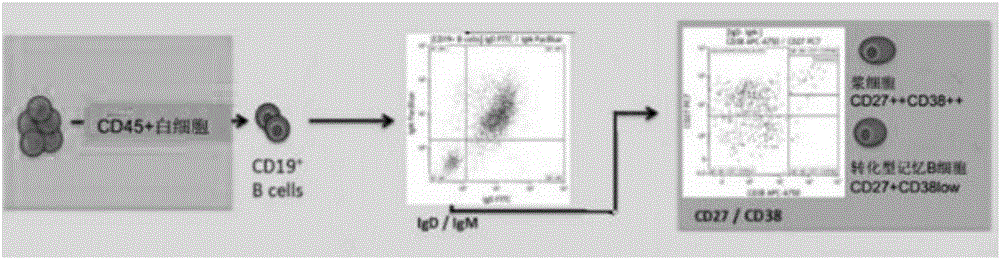
[0031] S1 Peripheral blood mononuclear cell isolation: take EDTA anticoagulated blood from the peripheral vein of Zika-infected patients in the acute stage, and use density gradient centrifugation to separate peripheral blood mononuclear cells, and aliquot 5×10 6 / tube, placed in liquid nitrogen for freezing;
[0032] S2 Plasma cell separation: Place the peripheral blood mononuclear cells frozen in step S1 in a 37°C water bath to dissolve, wash 3 times with PBS buffer, then add fluorescent-labeled antibody for staining, incubate at room temperature for 15 minutes in the dark, use PBS buffer After washing, 400 μl of PBS buffer was added to suspend the cells on a flow cytometer, and a single plasma cell was sorted out and frozen in a 96-well PCR plate; the antibodies were IgD, CD19, CD27, CD38, IgM, and CD45, and the labeled fluorescent For FITC, ECD, PC7, APC A750, PB and ...
Embodiment 2
[0037] Example 2. A specific operation step for rapid preparation of Zika virus-specific fully human monoclonal antibody
[0038] S1 Peripheral blood mononuclear cell isolation: take EDTA anticoagulated blood from the peripheral vein of Zika-infected patients in the acute stage, and use density gradient centrifugation to separate peripheral blood mononuclear cells, and aliquot 5×10 6 / tube and stored in liquid nitrogen.
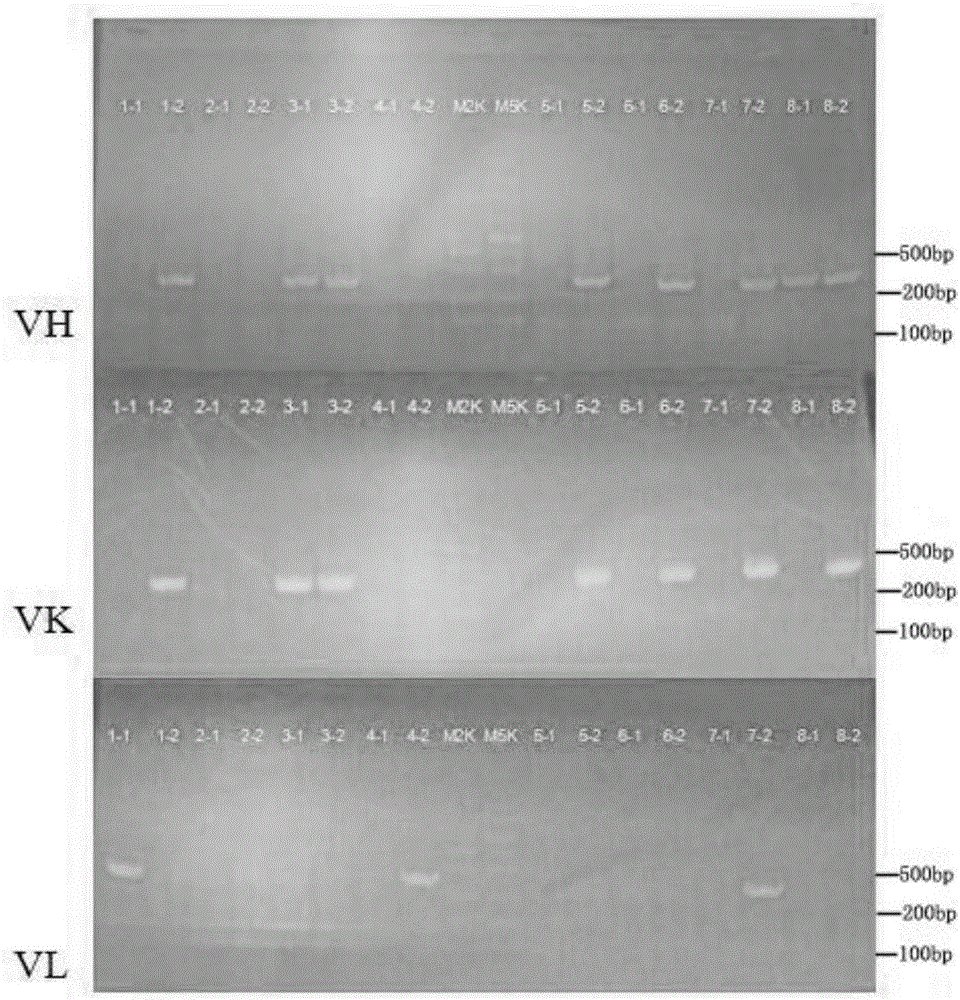
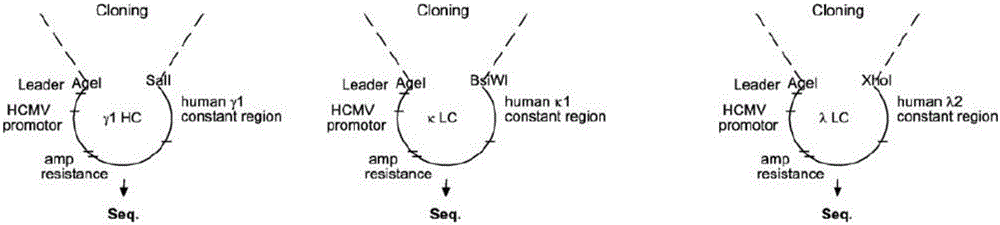
[0039] S2 Plasma cell separation: Place the peripheral blood mononuclear cells frozen in step S1 in a water bath at 37°C to dissolve, wash 3 times with PBS buffer, then add antibodies for staining, incubate at room temperature in the dark for 15 minutes, wash with PBS buffer , add 400 μl of PBS buffer to suspend the cells and put them on the flow cytometer, which is the Beckman Coulter MoFlo Astrios EQ ultra-high-speed flow cytometry sorting system, fluorescently labeled antibody staining: set up 9 analysis tubes, as shown in Table 1 , tubes 1-7 add corresp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com