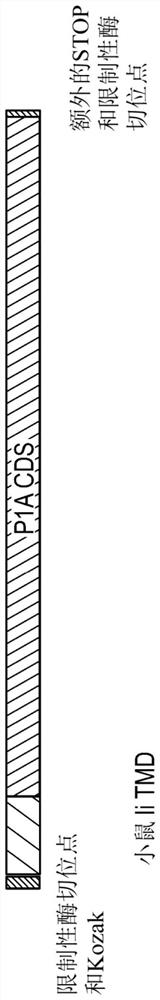
Viral vectors encoding cancer/testis antigens for use in a method of prevention or treatment of cancer
A technology of cancer antigen and carrier, which is applied in the direction of virus/phage, tumor rejection antigen precursor, and the introduction of foreign genetic material by carrier, which can solve the problems of reducing the risk of amplification and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Example 1: Induction of CD8 T cells against P1A by immunization with ChAdOx / MVA
[0185] P1A is a murine MAGE-type antigen that can be used to confirm the induction of CD8 responses against non-mutated MAGE-type antigens in syngeneic hosts and to induce protection against P1A-expressing tumors P815. It is a replacement for MAGE-A3 and NY-ESO-1.
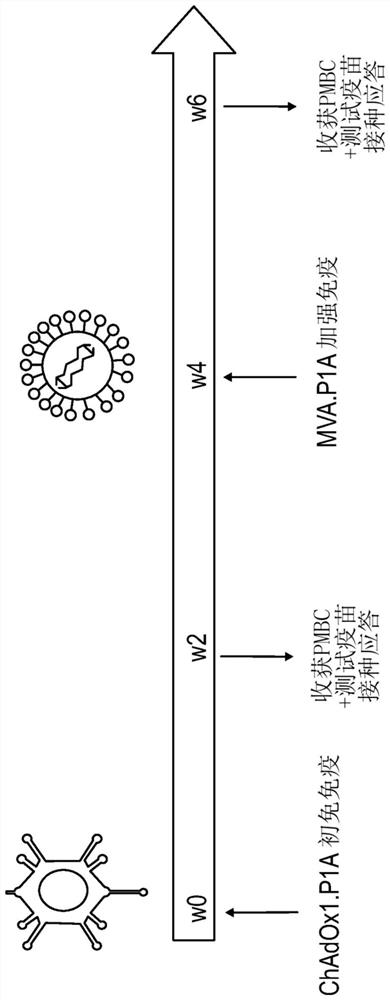
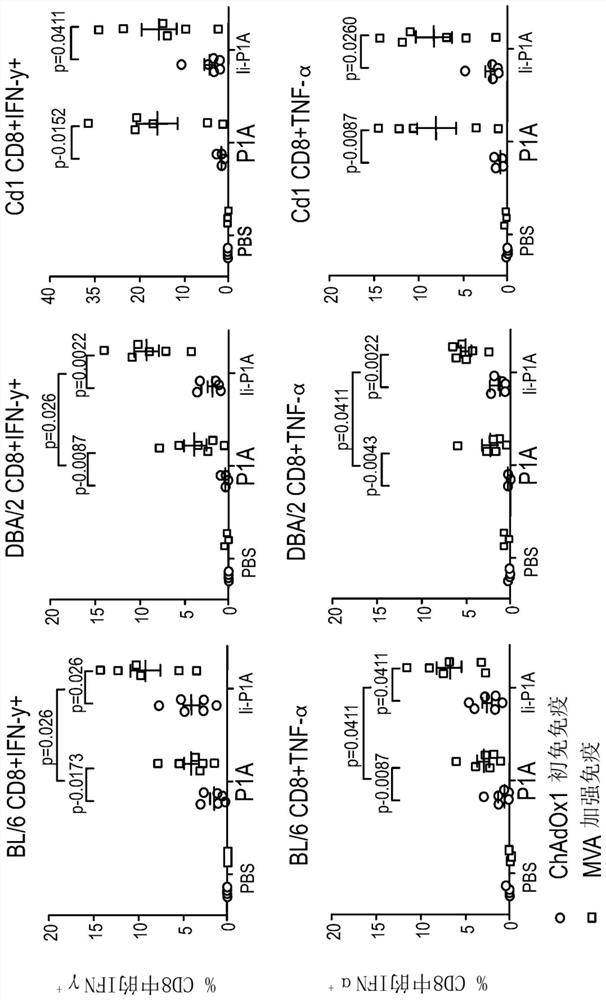
[0186] Figure 1B A schematic diagram of the prime-boost immunization of mice from which peripheral blood mononuclear cells (PBMC) were obtained and tested for vaccination response is shown. figure 2CD8 in mice vaccinated with ChAdOx1 / MVA viral vector encoding P1A is shown + T cell response. Timeline according to vaccination protocol (Figure 1) – BL / 6, DBA / 2 and CD1 mice were vaccinated with ChAdOx1.P1A or ChAdOx1.P1A-Ii (prime) followed by MVA.P1A (boost) 4 weeks later ). To test P1A-specific T cell responses, PMBCs were stimulated ex vivo with the P1A peptide pool and the percentage of cells producing type I cytokine...
Embodiment 2
[0188] Example 2: Evaluation of the protective efficacy of P1A-carrying ChAdOx1 and MVA viral vectors against tumor formation
[0189] As part of assessing the protective efficacy of the vaccine against tumor challenge, mice were injected subcutaneously with tumor cells in serum-free cell culture medium on one side. After the appearance of overt tumors, their growth was recorded and mice were euthanized once the tumor size reached 10 mm in either direction. Tumor volume was measured using the formula: length (mm) x width2 (mm) x 0.5. Survival curves were drawn according to the Kaplan-Meier method, and differences in survival were tested using the log-rank test. P values below 0.05 were considered significant.
[0190] In more detail, DBA / 2 mice were immunized with PBS, live L1210.P1A.B7-1 cells or given ChAdOx1-P1A(±Ii) / MVA-P1A prime-boost immunization at intervals of 4 weeks, and then on one side with 1.5x10 6 P815 cells or 1x10 6 15V4T3 cells were challenged by subc...
Embodiment 3
[0194] Example 3: Evaluation of Vaccine Therapeutic Efficacy of ChAdOx1 and MVA Viral Vectors Carrying P1A
[0195] Experiments are conducted to assess the therapeutic efficacy of vaccines. After the establishment of 15V4T3 tumors, mice were primed with ChAdOx vector from 5 days later, and then boosted with MVA vector on day 26. Vaccination regimens such as Figure 7 shown. Three different vaccination regimens were tested. Subcutaneously via the ventral side with 1x10 6 DBA / 2 mice were challenged with 15V4T3 cells, and then received 5 days after challenge: a single standard dose of ChAdOx-P1A (10 8 IU) / MVA-P1A (10 7 PFU) prime-boost vaccination, a single low-dose prime-boost vaccination (ChAdOx–10 7 IU, MVA–10 6 PFU), weekly alternating low-dose ChAdOx-P1A / MVA-P1A vaccination or weekly ChAdOx / MVA vaccination encoding the control antigen DPY. Growth and survival of 15V4T3 tumors in each treatment group were then monitored.
[0196] As shown in Figure 8, tumor growth ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com