Expression vector based on adenovirus AdC68 and construction method thereof
An expression vector and adenovirus technology, applied in the fields of biotechnology and virology, can solve the problems of cumbersome screening of positive clones, easy mutation of adenovirus genome, and increased genetic changes of adenovirus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1. Amplification of wild-type adenovirus AdC68 and its genome isolation
[0116] The wild-type adenovirus AdC68 purchased from ATCC was amplified in HEK293 cells, the supernatant of the virus was collected, and then purified by cesium chloride gradient centrifugation to obtain a large amount of wild-type adenovirus AdC68. Isolate the genome of adenovirus AdC68, digest it with Mfe I and Bgl II, and go through 1% agarose gel electrophoresis. The digested DNA band proves that the genome of AdC68 is complete and correct, see figure 1 .
Embodiment 2
[0117] Example 2, construction of replication-deficient adenovirus AdC68
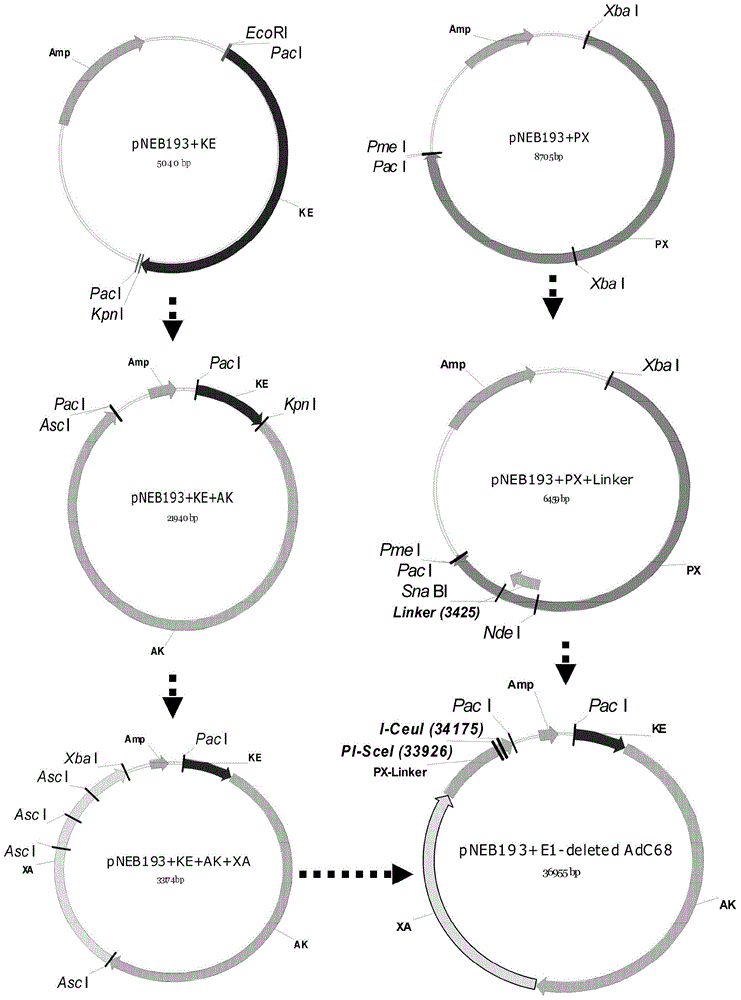
[0118] According to the analysis of the nucleotide sequence of the adenovirus AdC68 genome, it was divided into four fragments by using the properties of the restriction site ( FIG. 2A ), and then cloned into the pNEB193 vector respectively in sequence. Among them, the E1 part of the adenovirus AdC68 is connected with a Linker fragment containing the restriction sites I-Ceu I and PI-Sce I through the two restriction sites of SnaB I and Nde I, which will replace the about 3.5kb fragment of the E1 part . The identification of restriction endonucleases Xho I, Bgl II, Mfe I and Xma I proved that the constructed replication-defective adenovirus vector AdC68 was correct, as shown in Fig. 2B-C.
Embodiment 3
[0119] Example 3. Construction of the replication-deficient adenoviral vector pNEB193-E1-deleted-EGFP containing the reporter gene EGFP
[0120] PI-Sce I and I-Ceu I double digest the pShuttle-CMV-EGFP plasmid, recover the target fragment of CMV-EGFP from agarose gel, and connect it to the pAdC68- The E1-deleted vector was transformed into Stbl2 competent cells, the positive clones were screened, and the reporter gene CMV-EGFP was identified to be completely correct after enzyme digestion, as shown in Figure 3A-B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com