A kind of rabies vaccine and preparation method thereof
A rabies vaccine and adenovirus technology, applied in the fields of biotechnology and virology, can solve problems such as weakening the immune effect of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1. Cloning of rabies virus Gp coding region
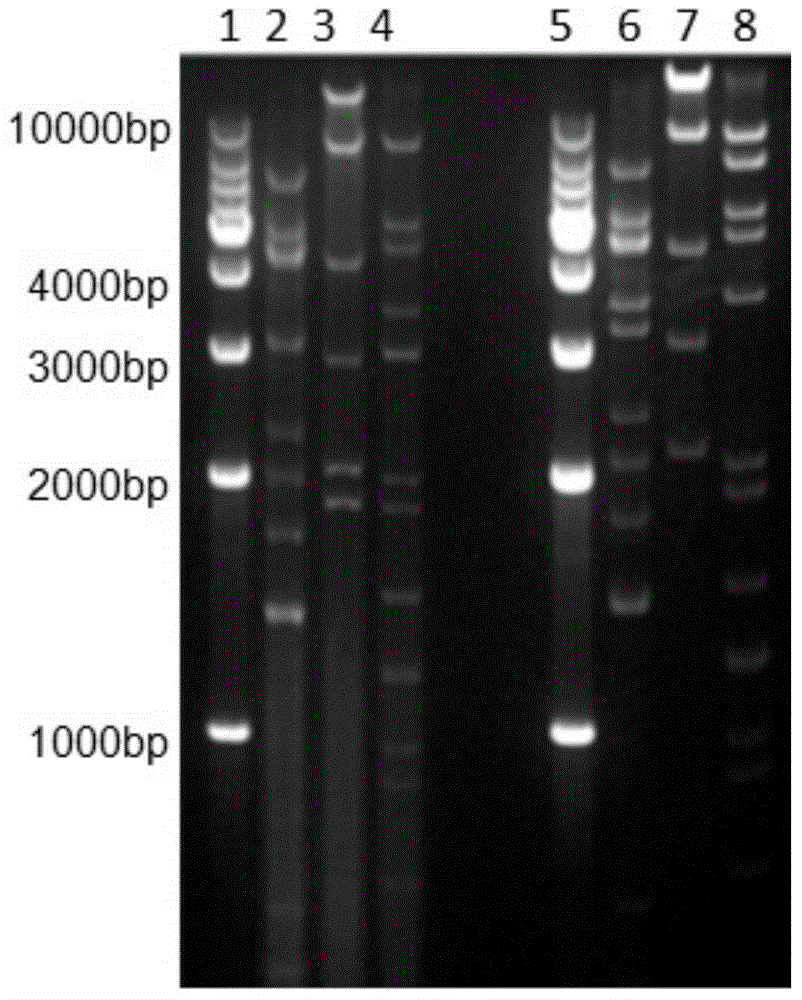
[0108] Agarose gel electrophoresis shows that the digestion of PUC57-0 / Gp is consistent with the expected size (bp), such as figure 2 ; Since Nhe I and Xba I are homologous enzymes, reverse cloning may occur when screening the recombinant shuttle plasmid pAdshuttle-CMV / gp. First, use Nhe I and Xba I to identify positive clones, and then use pAdshuttle-CMV / gp The single restriction site EcoRI in the region outside the target fragment was identified again, and the recombinant adenovirus shuttle vector pAdshuttle-CMV / Gp was cut with restriction enzymes, and the results were consistent with expectations. The sequencing results show that the obtained nucleic acid sequence is the same as the nucleic acid sequence after the optimized codon of the target fragment.
Embodiment 2
[0109] Example 2. Identification of recombinant adenovirus
[0110] PI-Sce I and I-Ceu I double digested pAdshuttle-CMV / gp and pAdC68, and ligated the above products with T4DNA ligase. After selecting the ampicillin resistant clones, restriction enzyme digestion to identify the plasmid pAdC68-Gp, such as image 3 , Consistent with the expected size. The sequencing results show that the obtained nucleic acid sequence is the same as the nucleic acid sequence after the optimized codon of the target fragment.
[0111] A large amount of recombinant adenovirus plasmid DNA was prepared, and the recombinant plasmid digested with Pac I was transfected into HEK293 cells. Obvious cytopathic changes appeared after 6-8 days. Plaques appear in the cells in the early stage of the disease, and gradually become larger, and in the later stage, they are in a net state, and finally all the cells float. Collect 20 bottles of 150ml cells that have been infected with the virus. The mixture of repeated...
Embodiment 3
[0112] Example 3. Determination of recombinant adenovirus infection titer and analysis of genetic stability
[0113] Infect HEK293 cells and liver cancer cell line Huh7 with different numbers of viruses, such as Figure 4 . After 24 hours of infection, plaques appeared in HEK293 cells (the amount of virus was 10 8 vps); and Huh7 cells after infection (with a viral load of 10 8 vps) has no significant difference with the control group, when the virus amount reaches 10 10 Plaques also appeared in vps and Huh7 cells. Pass the purified virus in HEK293 cells for 15 consecutive generations, collect and purify the virus in a small amount, and identify the genomes of the 5th and 15th generation viruses by restriction enzyme digestion. The results prove that the recombinant virus has no mutations and maintains the original virus infection. Ability, such as Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com