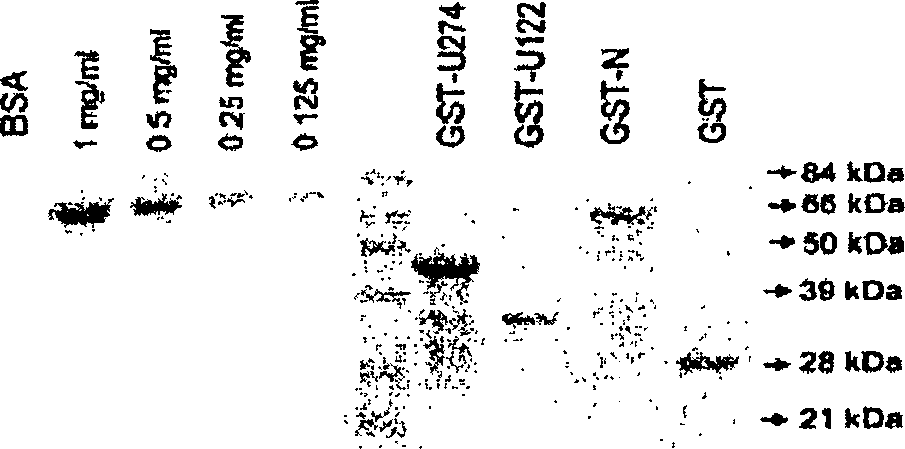Method of diagnosing SARS corona virus infection
A coronavirus and antibody technology, applied in the field of diagnosing SARS coronavirus infection, can solve problems such as time-consuming and difficult RNA extraction methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1: Use of SARS CoV protein as a diagnostic marker
[0126] Materials and methods
[0127] Materials: Unless otherwise specified, all reagents used in this study were purchased from Sigma (St. Louis, Mo.). All cell lines were purchased from the American Type Culture Collection (Manassas, Va.) and cultured at 37°C in 5% CO 2 , Dulbecco's modified Eagle medium, the medium contains 1g glucose / liter, 2mM L-glutamine, 1.5g sodium bicarbonate / liter, 0.1mM non-essential amino acids, 0.1mg streptavidin / ml , 100 U of penicillin and 5% fetal bovine serum (HyClone, South Logan, Utah).
[0128] Construction of plasmids: For transient expression in mammalian cells, the vector used was pXJ40HA for tagging the N-terminus of the protein with one hemagglutinin (HA) epitope (15); The vector used for tagging at the C-terminus of the protein is pXJ40-3-HA. For expression of glutathione S-transferase (GST) fusion proteins in bacteria, the gene was cloned in-frame with GST into pG...
Embodiment 2
[0151] Example 2: ELISA and immunochromatographic assays
[0152] Materials and methods
[0153] Recombinant proteins: Materials and methods used to obtain recombinant proteins are as described in Example 1 above. For a study, use Superdex TM Gst-U274 was further purified on an S-200 HR10 / 30 column on an AKTA Fast Protein Liquid Chromatography (FPLC) system (Amersham). The buffer used contained 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 6 M urea and 1 mM β-mercaptoethanol at a flow rate of 0.5 ml / min, and 1 ml of fragments were collected. Fragments 12 and 13 were joined together and dialyzed against phosphate buffered saline (PBS) overnight with at least 3 buffer changes.
[0154] Serum samples: 74 convalescent serum samples were collected from patients admitted to Tan Tock Seng Hospital or Singapore General Hospital. Ninety-one control sera were obtained from healthy local donors working at the Institute of Molecular and Cell Biology in Singapore. All samples were collected ...
Embodiment 3
[0166] Example 3: Evaluation of ELISA and Immunochromatographic Assays
[0167] determination
[0168] Materials and methods
[0169] Serum samples: Serum samples were collected from patients clinically suspected of SARS according to the WHO definition (18) and from patients admitted between March 18 and May 24, 2003, in three acute regional hospitals in Hong Kong. A total of 227 serum samples from these patients were assayed with the IF assay (9) and confirmed to have IF titers >1:10-1:2560 dilution. At the same time, 385 serum samples from 385 healthy donors collected in Hong Kong were used as controls. In addition, 1066 sera from healthy donors purchased from BioClinical Partner Inc. (Franklin, MA) were included in this study as additional healthy controls. Genelabs Diagnostics (GLD, Singapore) also used archived serum samples from various previous studies for disease control; this included 50 samples from patients with non-SARS-associated fever (identified as dengue fev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com