Method for detecting pig plague virus specific antibody and its ELISA reagent kit
An enzyme-linked immunoassay reagent and swine fever virus technology, which is applied in the direction of measuring devices, material analysis and instruments through observation of the impact on chemical indicators, can solve the problem of affecting the effect of attenuated live vaccination, the vaccine is invalid, and cannot produce Virus immunity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, the preparation of the enzyme-linked immunosorbent assay kit that detects classical swine fever virus specific antibody
[0020] The enzyme-linked immunosorbent assay kit of detection classical swine fever virus specific antibody of the present invention comprises following reagent:
[0021] 1) Enzyme-labeled swine fever virus single epitope-specific antibody working solution;
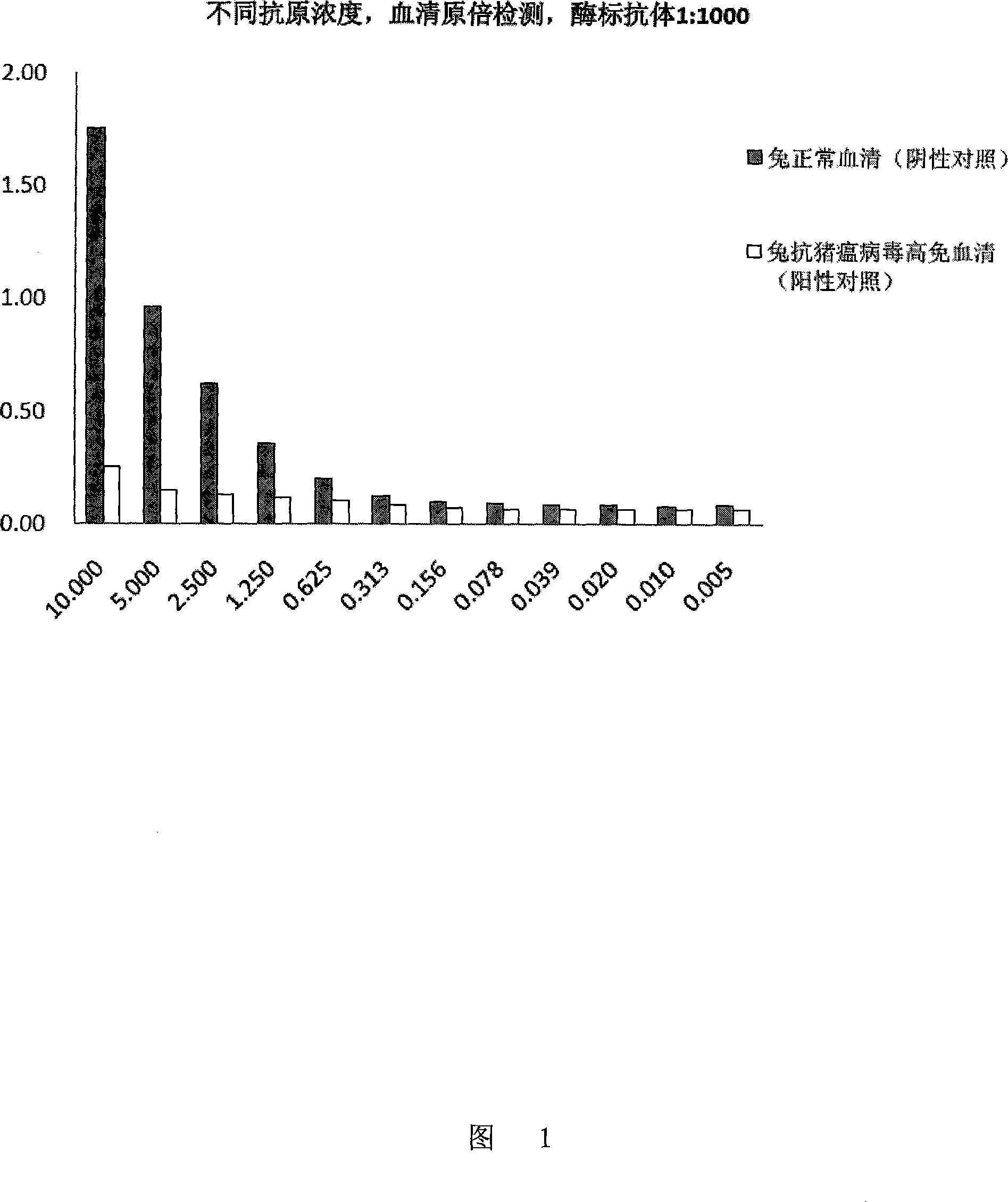
[0022] 2) Hog fever virus antigen working solution for coating: a polypeptide solution containing an epitope whose amino acid residue sequence is the sequence shown in Sequence 1; 10 μg / ml, the solvent is coating buffer, that is, 0.1M, pH9.6 bicarbonate Sodium buffer;
[0023] 3) ELISA plate
[0024] 4) Positive serum control: hyperimmune serum of rabbits with classical swine fever virus;
[0025] 5) Negative serum control: serum of healthy rabbits not exposed to CSFV;
[0026] 6) Washing solution: PBS buffer containing 0.05% Tween-20 by volume (PBS buffer contains 5.54g / L Na ...
Embodiment 2
[0072] Embodiment 2, the detection effect experiment of kit of the present invention
[0073] The enzyme-linked immunosorbent assay kit prepared in Example 1 is used to detect the serum containing classical swine fever virus antibody, and the specific steps are as follows:
[0074] 1. Add the coated antigen working solution prepared in Example 1 to the microtiter plate, 50 μl per well, and overnight at 4°C;
[0075] 2. Pour off the coating solution, and wash the microtiter plate once with the washing solution;
[0076] 3. Block the microtiter plate with blocking solution for 30 minutes;
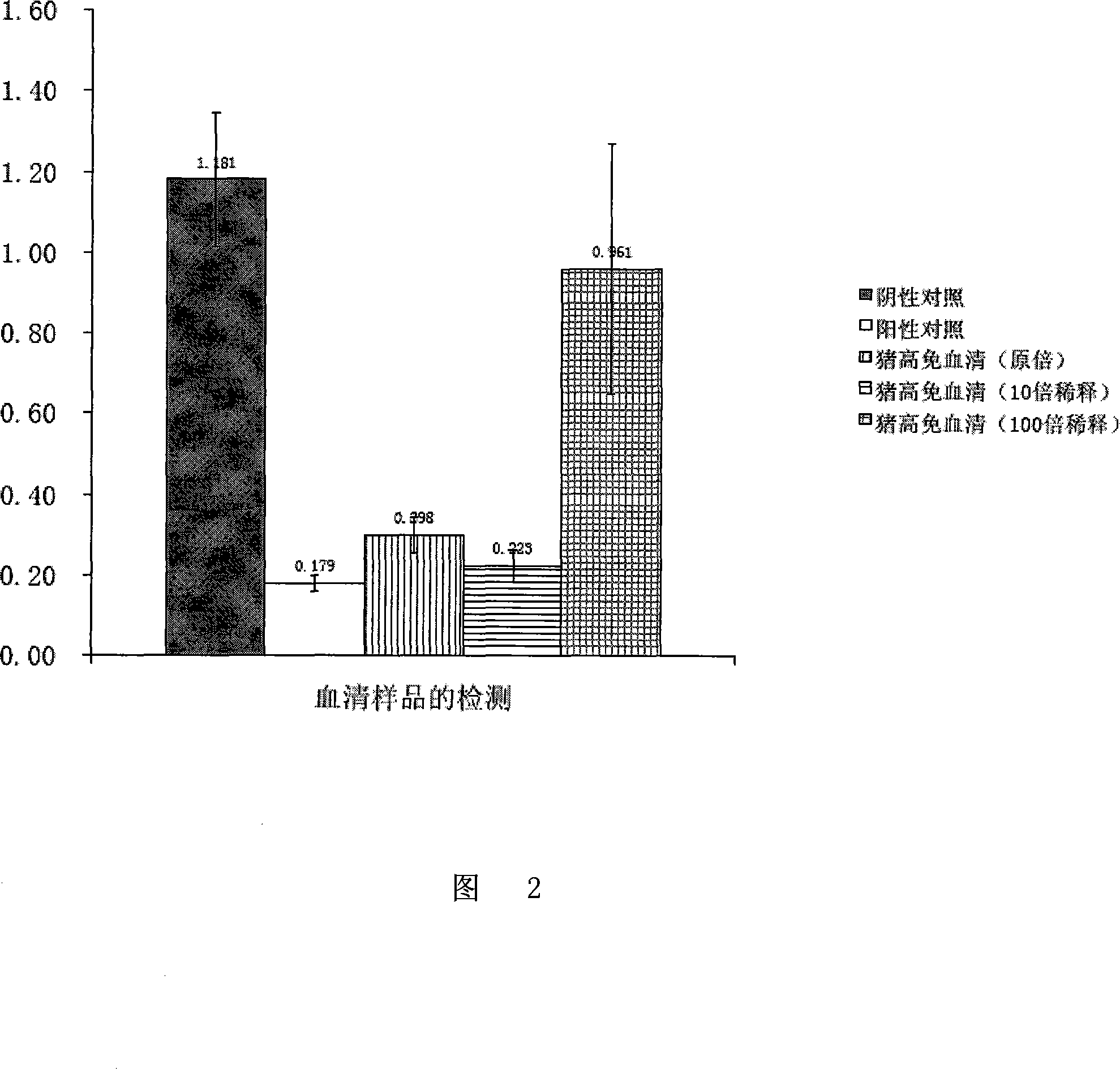
[0077] 4. Add 50 μl of positive serum control (primary swine fever virus rabbit hyperimmune serum) and negative serum control (no contact with CSFV) to each hole of the enzyme label plate after step 3 sealing. The healthy rabbit serum) and the swine fever virus-infected pig serum to be tested (purchased from the China Veterinary Drug Administration, including the original serum, 10-fold dil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com