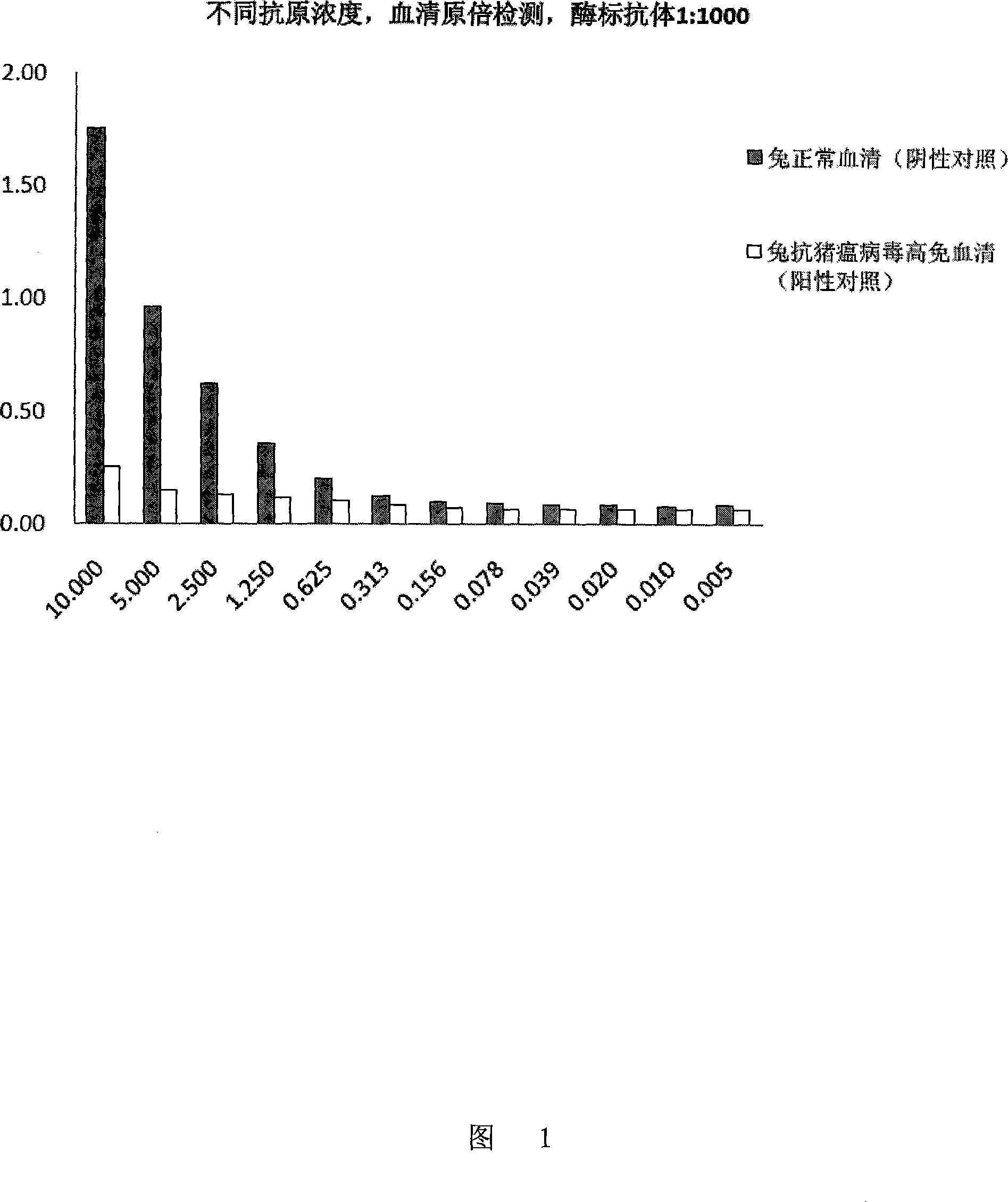
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Classical swine fever virus antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for detecting pig plague virus specific antibody and its ELISA reagent kit
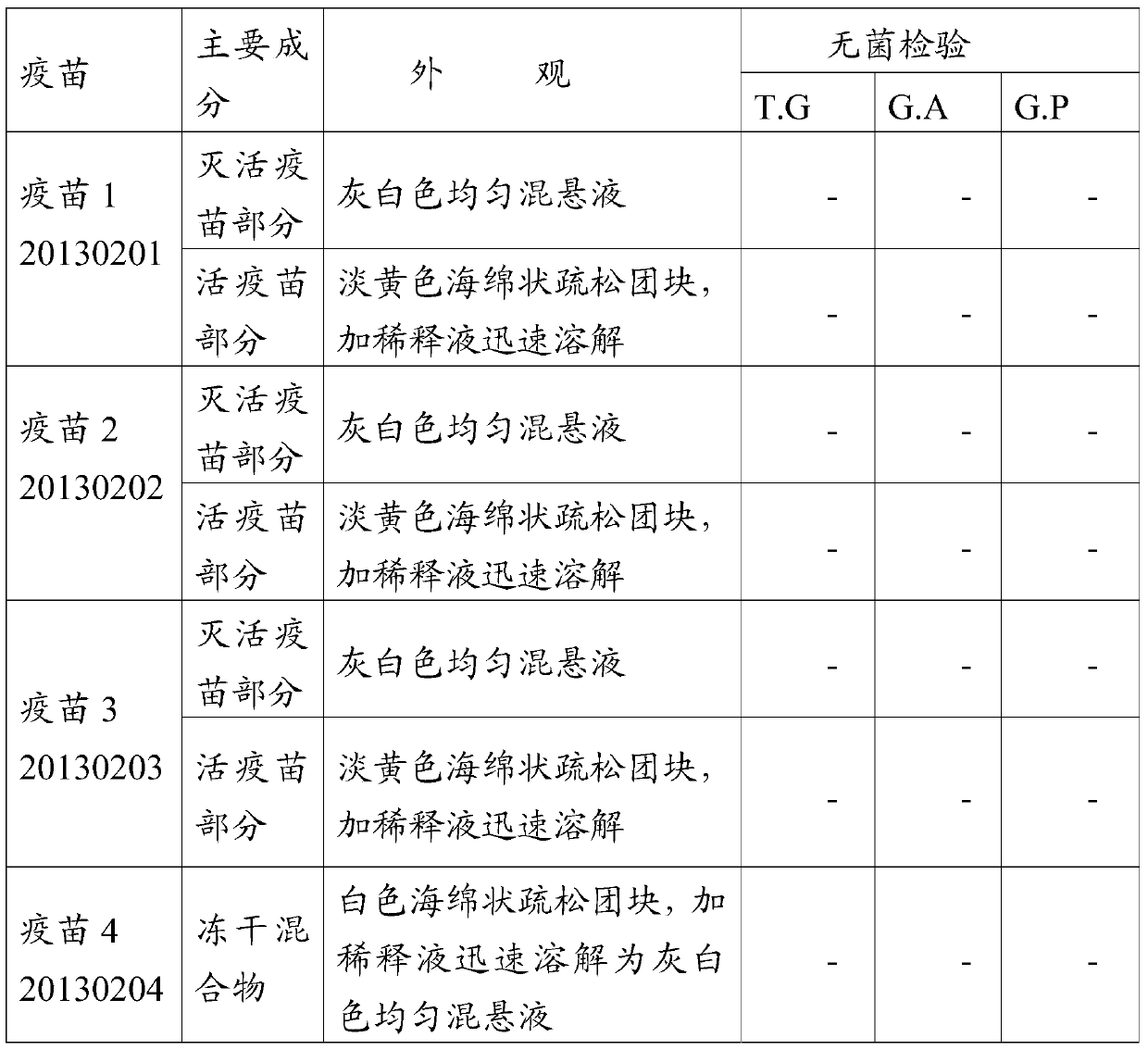
InactiveCN101144818AGuaranteed specificityGuaranteed Differential DiagnosisMaterial analysis by observing effect on chemical indicatorElisa kitSwine Fever Virus
The invention discloses a method for detecting the specific antibody of classical swine fever virus and a special ELISA kit thereof. The kit includes a classical swine fever virus antigen and an enzyme-labeled classical swine fever virus single-epitope specific antibody; the said swine fever virus antigen is a polypeptide containing one or more than one amino acid residue sequence described in sequence 1. The detection reagent of the classical swine fever virus specific antibody of the present invention can carry out effective detection to the classical swine fever virus specific antibody by solid-phase antigen competition ELISA (blocking method); The B cell epitope ensures the differential diagnosis, and the high degree of conservation of the epitope among various strains ensures the specificity of detection.
Owner:TSINGHUA UNIV +2
Swine fever vaccine heat-resistant freeze-drying protective agent, and preparation method and application thereof
The invention discloses a swine fever vaccine heat-resistant freeze-drying protective agent, and a preparation method and an application thereof. The heat-resistant freeze-drying protective agent comprises the following components: trehalose, polyvinylpyrrolidone (PVP), gelatin, sodium glutamate, D-sorbic alcohol, polypeptone, L-arginine, vitamin C and water for injection and is prepared by dissolving and degerming. The heat-resistant freeze-drying protective agent is mixed with swine fever virus antigen liquid according to the volume ratio of 1:(08-1.2) and is frozen and dried after being packaged to obtain swine fever vaccine. The prepared heat-resistant freeze-drying protective agent is simple in formula, is easy to prepare, is suitable for large-scale production and has good protection efficacy on the vaccine. The prepared vaccine has the characteristics of safety and sterility, stable quality, heat resistance and long saving time. The swine fever vaccine heat-resistant freeze-drying protective agent solves the problems that the vaccine is required to be frozen at a low temperature, is not convenient to store and the like in the transportation process. Through the swine fever vaccine heat-resistant freeze-drying protective agent, the swine fever vaccine can be saved for above 30 months at a temperature of 2-8 DEG C.
Owner:河南后羿生物工程股份有限公司
Colloidal gold immunochromatographic test paper detecting African swine fever virus antigen, preparation method and application thereof
The invention relates to colloidal gold immunochromatographic test paper detecting African swine fever virus antigen, a preparation method and application thereof, belonging to virus disease diagnosistechnologies and the field of animal quarantine. The test paper comprises a PVC bottom board, wherein a sample pad, a colloidal gold pad, a nitrocellulose membrane and a water absorption pad are fixed on the PVC bottom board in sequence, the colloidal gold pad is internally provided with one epitope of monoclonal antibody Mab1 of an anti-ASFV p30 antigen marked by colloidal gold, the surface of the nitrocellulose membrane is provided with a detection line and a quality control line, the detection line is designed for another epitope of monoclonal antibody Mab2 for ASFVp30 antigen, and the quality control line is a goat anti-mouse IgG antibody. The colloidal gold immunochromatographic test paper has the advantages of being convenient, rapid, sensitive and specific, can detect ASFV infectedcells directly, and is suitable for ASFV infected diagnosis, epidemiologic investigation and international trade quarantine for swine.
Owner:ROHI BIOTECH
Detection kit and application thereof
The invention provides a detection kit containing two anti-porcine circovirus 2 type monoclonal antibodies, two anti-classical swine fever virus monoclonal antibodies and / or two anti-highly pathogenic porcine reproductive and respiratory syndrome virus monoclonal antibodies; the kit can be applied for simultaneous detection of a porcine circovirus 2 type antigen, a classical swine fever virus antigen and / or a highly pathogenic porcine reproductive and respiratory syndrome virus antigen with non-diagnostic purpose, moreover, the detection sensitivity of the kit for simultaneous detection of two or three kinds of viruses is higher than that for the detection of single virus, and false positive results are avoided.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Vaccine composition, preparation method and application thereof
ActiveCN103908665AReduce the number of vaccinationsIncrease costAntibacterial agentsBacterial antigen ingredientsDiluentWater soluble
The invention relates to a vaccine composition, which contains classical swine fever virus antigen, porcine circovirus type 2 antigen, mycoplasma hyopneumoniae antigen and a vaccine adjuvant. The invention also provides a method for preparing the vaccine composition, a water-soluble vaccine adjuvant is used for preparing a mixture of the porcine circovirus type 2 antigen and mycoplasma hyopneumoniae antigen, and the mixture is taken as a diluent for diluting classical swine fever virus antigen. The vaccine composition has the advantages of simple preparation method, high titer content of the vaccine, and convenient and fast immunization, one time immunization can simultaneously preventing or treating swine fever, porcine circovirus type 2 antigen and mycoplasma hyopneumoniae, immunization cost is reduced, the immunization program is saved, and the application is more economic and reliable.
Owner:PU LIKE BIO ENG
Vaccine composition for classical swine fever from plant and manufacturing method thereof
ActiveUS20180305710A1Improve efficiencyImprove securitySsRNA viruses positive-senseViral antigen ingredientsCelluloseAntigen
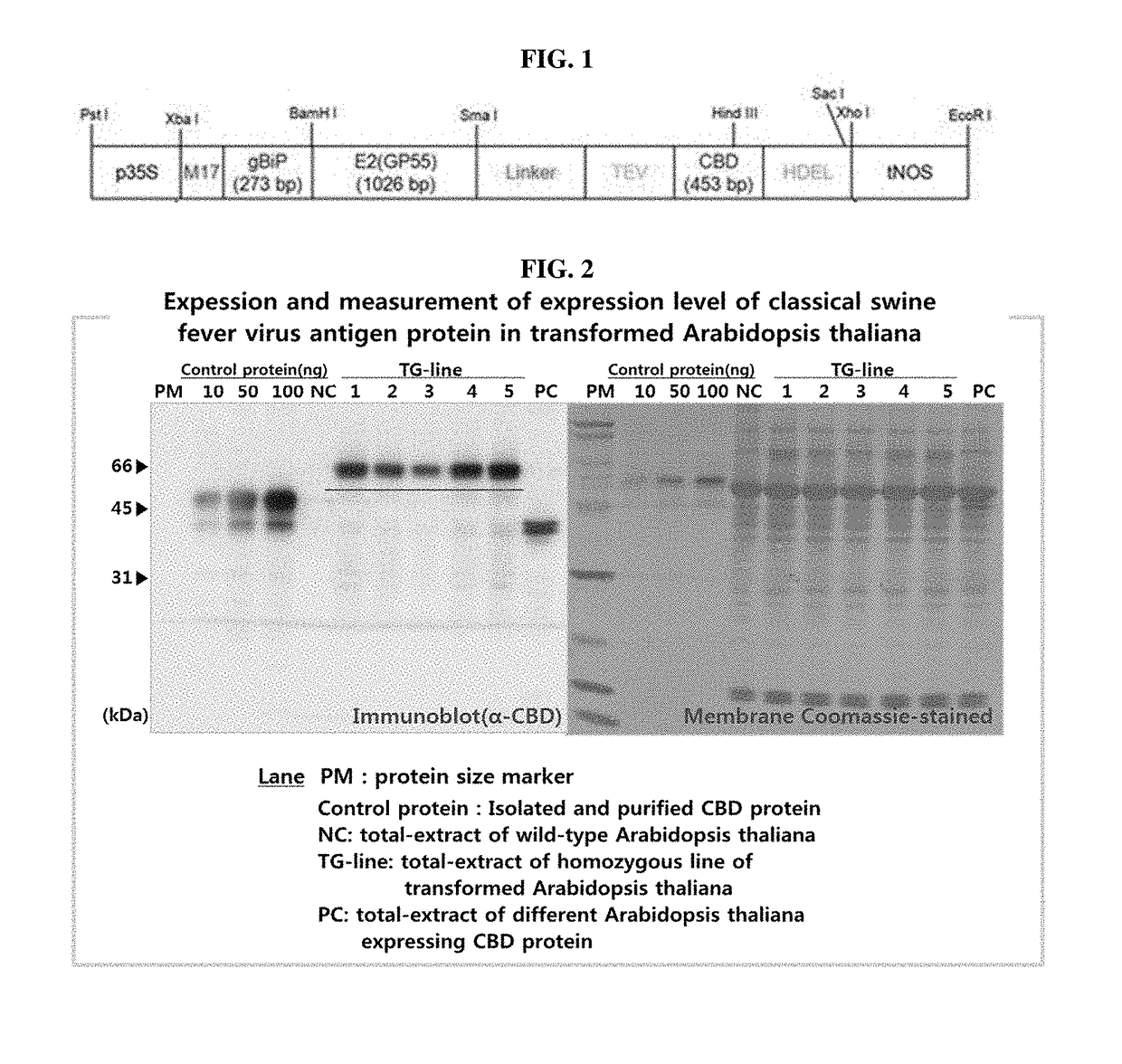
A recombinant vector for transforming a plant, a plant transformed with the recombinant vector, a plant-made classical swine fever virus antigen pmE2 protein expressed in the plant and uses thereof is provided. By using a recombinant vector having a polynucleotide encoding a GP55 protein of CSFV according to the present invention; and a polynucleotide encoding a cellulose-binding domain protein; and a plant transformed with the recombinant vector, a plant-made classical swine fever virus antigen pmE2 protein may be produced with high efficiency, and has higher safety and stability than those achieved by other production methods. Also, since the plant-derived classical swine fever virus antigen protein pmE2 has a cellulose-binding domain (CBD) protein, it may be usefully used as an effective marker to determine a virus exposure pathway and an antibody producing pathway.
Owner:BIOAPPL +1
African swine fever virus (ASFV) antigen detection fluorescent microsphere immunoassay test strip, preparation method and application
PendingCN110196323AEasy to useSimple and fast operationBiological material analysisBiological testingSlurryQuarantine
The invention discloses an African swine fever virus (ASFV) antigen detection fluorescent microsphere immunoassay test strip, which comprises a PVC bottom plate. A sample pad, a fluorescent pad, a nitrocellulose membrane and an absorbent pad are sequentially fixed on the PVC bottom plate; the fluorescent pad contains a monoclonal antibody Mab1 fluorescently labeled with an epitope of an anti-ASFVp30 antigen; the surface of the nitrocellulose membrane is marked with a detection line and a quality control line, the detection line is a monoclonal antibody Mab2 for another epitope of the ASFV p30antigen, and the quality control line is a goat anti-mouse IgG antibody. The test strip is suitable for detection on the ASFV p30 antigen in peripheral blood, serum, saliva and tissue (lung and spleen) polishing slurry of infected pigs, ASFV infection is diagnosed in a short time, and the test strip is particularly suitable for on-site ASFV infection diagnosis, epidemiological investigation and international trade quarantine inspection of pigs; and a result is directly displayed in collaboration with an immunofluorescence analyzer, the operation is quick and simple, the accuracy is high, andthe practicability is strong.
Owner:YANGZHOU UNIV
Vaccine composition, preparation method and application thereof
ActiveCN104511015AImprove immunitySatisfactory immune effectAntibacterial agentsAntiviralsImmune effectsAntigen
The invention provides a vaccine composition including a hog cholera virus antigen in an immunizing dose, a porcine pseudorabies virus antigen in an immunizing dose, and a haemophilus parasuis antigen in an immunizing dose. The vaccine composition is safe to use, is controllable in quality and is excellent in an immune effect. The vaccine composition can achieve a challenge protective rate being 80-100%. The vaccine composition, when being used for immunizing piglets in 0-3 day ages in a nasal-dropping manner and for immunizing piglets in 50 day ages in a muscle injection manner, can achieve a satisfying immune effect.
Owner:PU LIKE BIO ENG
Vaccine composition, and preparation method and application thereof
ActiveCN104288762ALow costReduce the number of vaccinationsAntibacterial agentsAntiviralsDiseaseHaemophilus
The invention provides a vaccine composition. The vaccine composition contains an immune amount of classical swine fever virus antigens, an immune amount of Haemophilus parasuis antigens, an immune amount of type 2 porcine circovirus antigens and a veterinarily acceptable carrier. The vaccine composition can effectively prevent and treat swine fever, the porcine circovirus disease and the Haemophilus parasuis disease, can reach double single vaccine injection immunization effect through one time injection, and has the advantages of few side reactions, long immune period, short time and less labor consumption.
Owner:PU LIKE BIO ENG
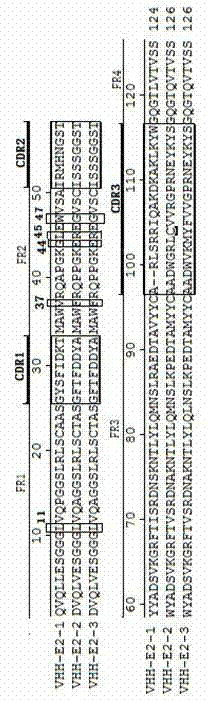
Bactrian camel source C-strain E2 VHH and application
ActiveCN103467599ASame antigen binding abilityBacteriaImmunoglobulins against virusesClassical swine fever virus CSFVCamelus bactrianus
The invention discloses bactrian camel source C-strain E2 VHH and application. The bactrian camel source C-strain E2 VHH and the application aim at solving the problem that production and preparation of high-purity high-appetency classical swine fever virus antibodies can not be obtained in the existing classical swine fever virus antigen diagnostic reagent research and development process. The bactrian camel source C-strain E2 VHH are characterized by being provided with a nucleotide sequence: SEQIDNO.1, SEQIDNO.2 or SEQIDNO.3. The invention further provides the application of the bactrian camel source C-strain E2 VHH in preparing a classical swine fever virus antigen testing diagnostic reagent. The bactrian camel source C-strain E2 VHH and the application have the advantages that the VHH coded sequence of C-strain antigen protein E2 is obtained for the first time through a Bactrian camel, ELISA experimental results show that the recombination VHH expressed in E.coli have the same antigen binding capacity as classical swine fever virus polyclone antiserum, and therefore the recombination VHH can replace the classical swine fever virus polyclone antiserum to be used for researching the classical swine fever virus antigen diagnostic reagent.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant baculovirus with surface displaying African swine fever virus antigen P30 protein, preparation method and application thereof
ActiveCN114262381AFacilitate successful transferStrong specificityViral antigen ingredientsMicroorganism based processesAntigenAfrican swine fever virus Antigen
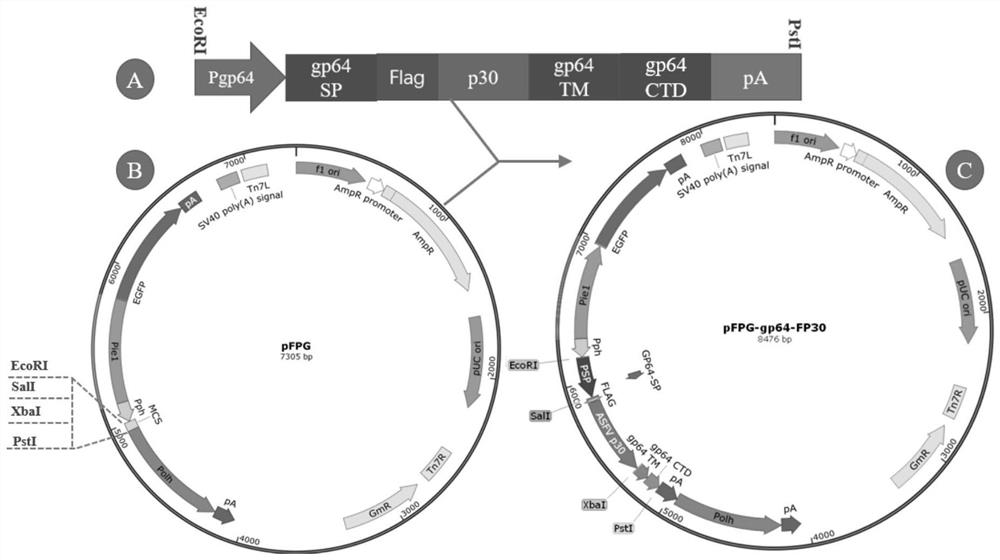
The invention discloses a recombinant baculovirus for displaying African swine fever virus antigen P30 protein on the surface as well as a preparation method and application of the recombinant baculovirus. A gene sequence of P30 is inserted between an N-terminal signal peptide SP sequence of baculovirus envelope glycoprotein gp64 and a fusion structural domain TMD to construct a fusion gene gp64-Fp30, then the fusion gene gp64-Fp30 is connected with a recombinant vector pFPG to construct a recombinant expression vector pFPG-gp64-Fp30, then the pFPG-gp64-Fp30 is converted into DH10Bac cells to obtain recombinant Bacmid plasmids, finally, the recombinant Bacmid plasmids are transfected into Sf9 cells, and the recombinant baculovirus envelope glycoprotein gp64 fusion protein is obtained. The recombinant baculovirus with the surface displaying the African swine fever virus antigen P30 protein is obtained. The recombinant P30 protein produced by using the recombinant baculovirus has a conformation similar to that of natural protein, can be induced to generate a serum antibody with excellent sensitivity and specificity, can also realize efficient and rapid expression of the P30 antigen, and has a wide application prospect in preparation of ASFV diagnostic reagents or ASF vaccines.
Owner:YANGZHOU UNIV
Method for detecting pig plague virus specific antibody and its ELISA reagent kit
InactiveCN101144818BGuaranteed specificityGuaranteed Differential DiagnosisMaterial analysis by observing effect on chemical indicatorClassical swine fever virus CSFVEpitope specificity
The present invention discloses a swine fever virus specificity antibody testing method and the special enzyme-linked immunity reagent kit thereof. The reagent kit includes a swine fever virus antigen and an enzyme labeled swine fever virus single location-indicating specificity antibody. The swine fever virus antigen is a polypeptide containing one or more aminophenol residue sequences of the sequence 1. The test reagent for the swine fever virus specificity antibody of the present invention can conduct effective test on the swine fever virus specificity antibody by the solid phase antigen competition enzyme-linked immunity method (interruption method). The cell B location-indicating of the selected swine fever virus strain ensures the differential diagnosis, and the high conservative ofthe location-indicating among the virus stains can ensure the testing specificity simultaneously.
Owner:TSINGHUA UNIV +2
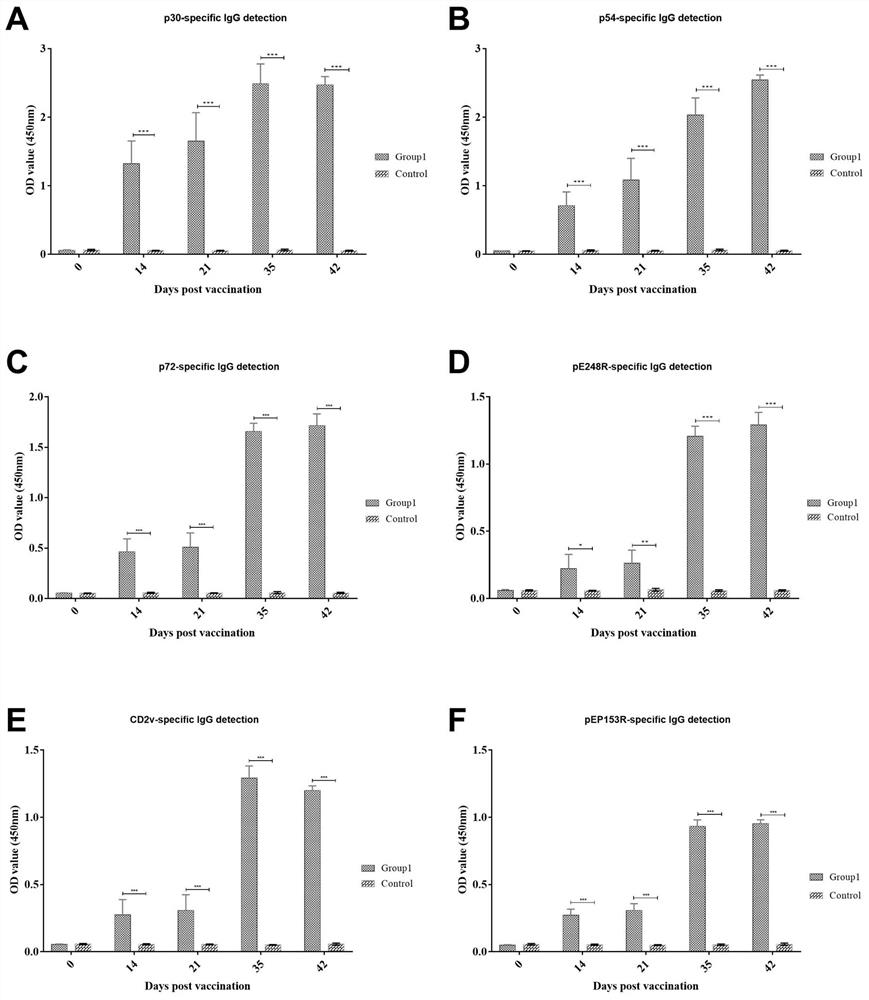
Recombinant African swine fever antigen cocktail vaccine containing intramolecular adjuvant and application thereof
PendingCN114377121AHigh expressionEasy to purifyViral antigen ingredientsAntibody mimetics/scaffoldsAdjuvantAfrican swine fever
The invention relates to a recombinant African swine fever antigen'cocktail 'vaccine containing an intramolecular adjuvant and application of the recombinant African swine fever antigen'cocktail' vaccine, and belongs to the field of biotechnological pharmacy, and the'cocktail 'vaccine contains three recombinant African swine fever virus antigen fusion proteins, namely OprI-p30-modified p54-TT, an N-segment amino acid sequence-TT of OprI-p72 epitope-pE248R and an N-segment amino acid sequence of OprI-CD2v and a C-segment amino acid sequence-TT of pEP153R. The invention provides expression and purification methods of the three recombinant African swine fever virus antigen fusion proteins, and component proportions and adjuvant compatibility for preparing the cocktail vaccine. The cocktail vaccine prepared by the invention can stimulate an organism to generate a neutralizing antibody and specific cellular immune response, and can be used as a subunit vaccine for preventing African swine fever.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Swine fever vaccine heat-resistant freeze-drying protective agent, and preparation method and application thereof
The invention discloses a swine fever vaccine heat-resistant freeze-drying protective agent, and a preparation method and an application thereof. The heat-resistant freeze-drying protective agent comprises the following components: trehalose, polyvinylpyrrolidone (PVP), gelatin, sodium glutamate, D-sorbic alcohol, polypeptone, L-arginine, vitamin C and water for injection and is prepared by dissolving and degerming. The heat-resistant freeze-drying protective agent is mixed with swine fever virus antigen liquid according to the volume ratio of 1:(08-1.2) and is frozen and dried after being packaged to obtain swine fever vaccine. The prepared heat-resistant freeze-drying protective agent is simple in formula, is easy to prepare, is suitable for large-scale production and has good protection efficacy on the vaccine. The prepared vaccine has the characteristics of safety and sterility, stable quality, heat resistance and long saving time. The swine fever vaccine heat-resistant freeze-drying protective agent solves the problems that the vaccine is required to be frozen at a low temperature, is not convenient to store and the like in the transportation process. Through the swine fever vaccine heat-resistant freeze-drying protective agent, the swine fever vaccine can be saved for above 30 months at a temperature of 2-8 DEG C.
Owner:河南后羿生物工程股份有限公司
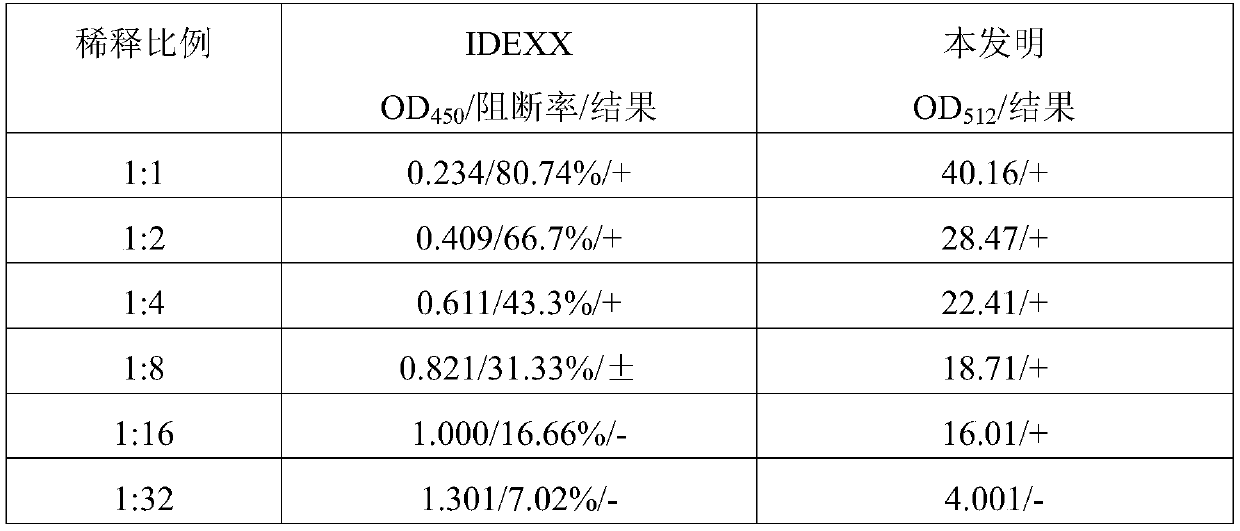
Test strip card for quantitative detection of classical swine fever virus antibody with double antigen sandwich and double detection line
ActiveCN108957002BHigh sensitivityReal-time quantitative detectionBiological material analysisBiological testingEscherichia coliClassical swine fever virus CSFV
The invention belongs to the field of animal immune detection, and in particular relates to a test paper card for quantitative detection of swine fever virus antibody with double antigen sandwiches and double detection lines. The test card includes sample pad, classical swine fever virus E2 protein gold standard pad, rabbit IgG gold standard pad, absorbent pad, double detection line coated with classical swine fever virus E2a protein, and nitrocellulose membrane of goat anti-rabbit IgG quality control line and PVC rubber sheet; colloidal gold adopts double-antigen sandwich and double detection line; the classical swine fever virus gold standard pad is coated with the protein antigen secreted by Escherichia coli BL21 / pE2 with the preservation number CCTCC NO: M2018060. E2 antigen and gold-labeled rabbit IgG; the nitrocellulose membrane is coated with the CSFV E2a antigen constructed from the protein antigen secreted by Escherichia coli BL21 / pE2a with the preservation number CCTCC NO: M2018059, constituting a double detection line area and The quality control line region is composed of goat anti-rabbit IgG.
Owner:武汉观锐生物科技有限公司
Hog fever cell-adapted virus, hog fever virus live vaccine prepared therefrom and preparation method thereof
ActiveCN103571797BShort breeding timeMany harvestsInactivation/attenuationMicroorganism based processesSwine Fever VirusVaccine virus
Owner:PU LIKE BIO ENG
Recombinant African swine fever virus antigen cocktail vaccine and application thereof
The invention relates to a recombinant African swine fever virus antigen'cocktail 'vaccine and application thereof, and belongs to the field of biotechnological pharmacy, the'cocktail' vaccine is prepared by compatibility of three components including an N-segment amino acid sequence of recombinant protein p30-modified p54, p72 epitope-pE248R, an N-segment amino acid sequence of CD2v and a C-segment amino acid sequence of pEP153R with an adjuvant. The invention provides expression and purification methods of the three recombinant proteins, and proportions and adjuvants of the recombinant proteins for preparing the cocktail vaccine. The cocktail vaccine for immunizing pigs can stimulate the body to generate neutralizing antibodies and cellular immunity, and is an African swine fever subunit vaccine with a very good application prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A kind of vaccine composition and its preparation method and application
ActiveCN103908665BReduce the number of vaccinationsIncrease costAntibacterial agentsBacterial antigen ingredientsWater solublePorcine circovirus
The invention relates to a vaccine composition, which contains classical swine fever virus antigen, porcine circovirus type 2 antigen, mycoplasma hyopneumoniae antigen and a vaccine adjuvant. The invention also provides a method for preparing the vaccine composition, a water-soluble vaccine adjuvant is used for preparing a mixture of the porcine circovirus type 2 antigen and mycoplasma hyopneumoniae antigen, and the mixture is taken as a diluent for diluting classical swine fever virus antigen. The vaccine composition has the advantages of simple preparation method, high titer content of the vaccine, and convenient and fast immunization, one time immunization can simultaneously preventing or treating swine fever, porcine circovirus type 2 antigen and mycoplasma hyopneumoniae, immunization cost is reduced, the immunization program is saved, and the application is more economic and reliable.
Owner:PU LIKE BIO ENG
African swine fever virus antigen recombinant tandem epitope protein, construction method and application thereof, and antibody ELISA kit
PendingCN114773483AHigh expressionSolubleAntibody mimetics/scaffoldsVirus peptidesElisa kitInclusion bodies
The invention discloses an African swine fever virus antigen recombinant tandem epitope protein, which is formed by connecting B cell epitopes of an African swine fever virus CD2v protein and an MGF360-505R protein in series through a flexible peptide, and the tandem epitope protein has higher expression quantity and inclusion body content, has certain solubility, has reactivity with a serum antibody, and can be used for antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection. The invention also discloses a construction method of the African swine fever virus antigen recombinant tandem epitope protein, an application of the African swine fever virus antigen recombinant tandem epitope protein in antibody ELISA detection and an antibody ELISA detection kit. According to the application and the antibody ELISA detection kit, the specificity, repeatability and stability are good, the sensitivity is high, serum samples generated after ASFV wild type and CD2v gene deletion type strain immunization can be accurately recognized, a certain reference value is achieved for ASF detection, and a serological detection method based on conservative epitopes is expected to provide a wider diagnosis range.
Owner:HUNAN AGRICULTURAL UNIV +1
Bactrian camel source C-strain E2 VHH and application
ActiveCN103467599BSame antigen binding abilityBacteriaImmunoglobulins against virusesClassical swine fever virus CSFVCamelus bactrianus
The present invention disclosed a dual -peak camel source anti -swine fever rabbing weak toxic vaccine plant E2 antigen heavy chain antibody VHH and use to solve the existing existing swine fever virus antigen diagnostic reagent research and development process.The production and preparation of swine fever virus antibodies.A twin -peak camel source anti -swine fever rabbit, weak poisonous vaccine, E2 antigen heavy chain antibody VHH, the antibody has nucleotide sequence: SEQ ID No.1, SEQ ID No.2 or SEQ ID No.3.The Shuangfeng Camel anti -swine fever rabbit bunocide vaccine plant E2 antigen heavy chain antibody VHH is used in the application of the application of swine fever virus antigen diagnostic reagents.The invention of the present invention is characterized by the first time obtaining the use of Shuangfeng camel screening to obtain the VHH antibody coding sequence of the VHH antibody coding of the antigen E2 of the anti -poison vaccine plant of the swine fever virus.Dopoline antibody has the same antigen -binding capacity, which can replace swine fever virus Dopolon antibodies in the study of swine fever virus antigen diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
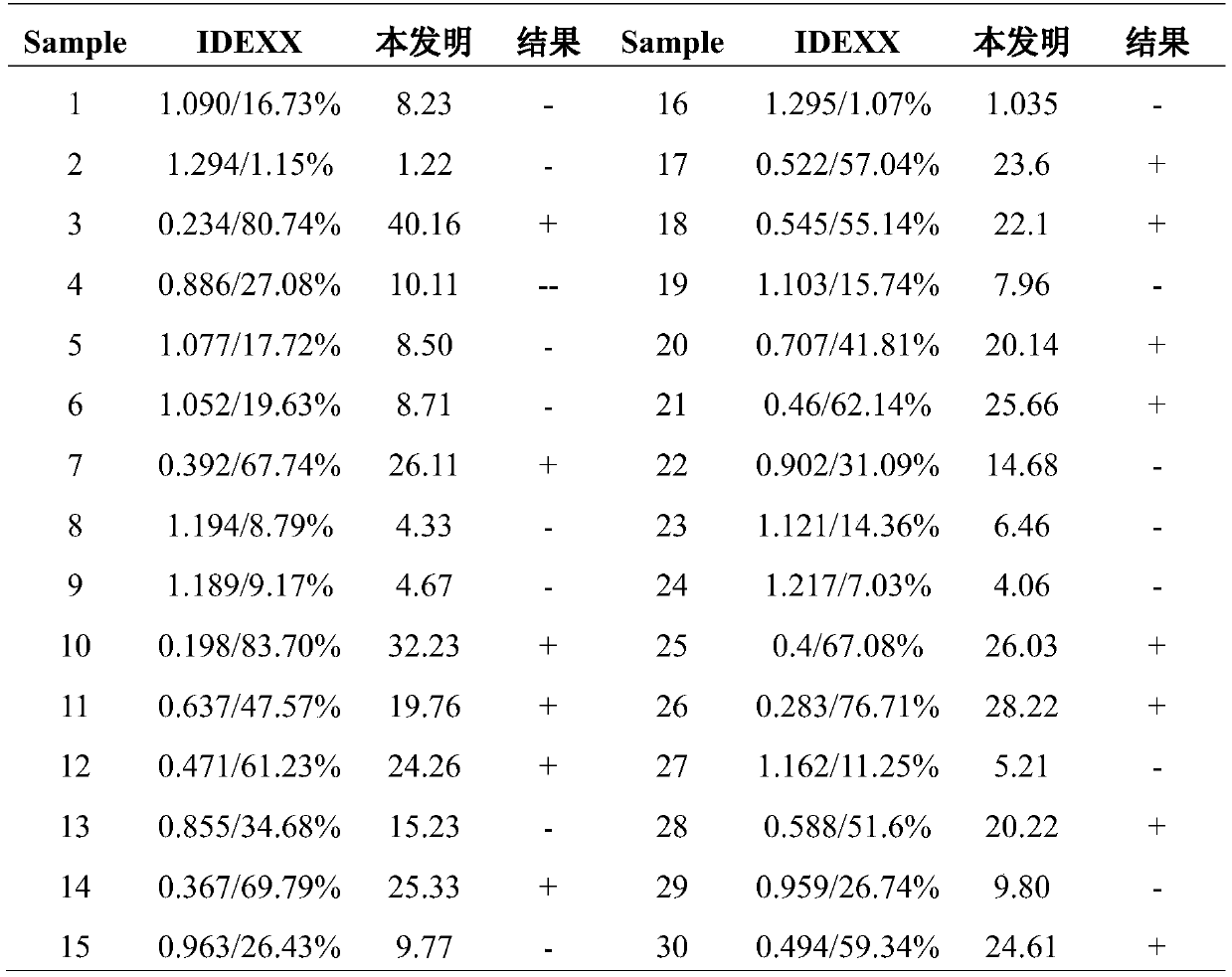
Swine fever virus antibody detection kit
InactiveCN110554199AHigh detection sensitivityImprove featuresBiological material analysisBiological testingPreservativeLatex particle
The invention discloses a swine fever virus antibody detection kit, and belongs to the technical field of biological detection. The swine fever virus antibody detection kit comprises a reagent R1 anda reagent R2, wherein the reagent R1 is a buffer solution with the pH value of 6.0-8.0 and comprises a stabilizer, a polymer accelerator, a surfactant and a preservative; the reagent R2 is a swine fever virus antigen latex reagent and comprises a stabilizer, swine fever virus E2 antigen latex particles, a preservative and a buffer solution. The kit disclosed by the invention has the advantages ofhigher detection sensitivity and specificity, good stability, simplicity in operation and short reaction time, is suitable for a full-automatic biochemical analyzer, a special protein instrument and aspectrophotometer, does not generate precipitate after reaction, and facilitates cleaning of a reaction vessel.
Owner:QINGDAO HARRENS INSPECTION & TESTING INC +1
A kind of vaccine composition and its preparation method and application
ActiveCN104288762BLow costReduce the number of vaccinationsAntibacterial agentsAntiviralsAntigenDisease
Owner:PU LIKE BIO ENG
Vaccine composition for classical swine fever from plant and manufacturing method thereof
ActiveUS10344293B2Improve efficiencyImprove securitySsRNA viruses positive-senseViral antigen ingredientsCelluloseAntigen
A recombinant vector for transforming a plant, a plant transformed with the recombinant vector, a plant-made classical swine fever virus antigen pmE2 protein expressed in the plant and uses thereof is provided. By using a recombinant vector having a polynucleotide encoding a GP55 protein of CSFV according to the present invention; and a polynucleotide encoding a cellulose-binding domain protein; and a plant transformed with the recombinant vector, a plant-made classical swine fever virus antigen pmE2 protein may be produced with high efficiency, and has higher safety and stability than those achieved by other production methods. Also, since the plant-derived classical swine fever virus antigen protein pmE2 has a cellulose-binding domain (CBD) protein, it may be usefully used as an effective marker to determine a virus exposure pathway and an antibody producing pathway.
Owner:BIOAPPL +1
Genetic engineering subunit oral vaccine for African swine fever virus
ActiveCN114456240AGood effectBacteriaVirus peptidesClassical swine fever virus CSFVStaphylococcus lactis
The invention provides an African swine fever virus genetic engineering subunit oral vaccine, which comprises genetic engineering lactococcus lactis for recombinant expression of African swine fever virus antigen protein, and the antigen protein is ASFV p30 protein with an amino acid sequence of SEQ ID NO: 1, ASFV p54 protein with an amino acid sequence of SEQ ID NO: 3 or ASFV p72 protein with an amino acid sequence of SEQ ID NO: 5. The antigen protein is connected with an Escherichia coli heat-labile enterotoxin B subunit LTB gene protein. The African swine fever virus genetic engineering subunit oral vaccine can effectively stimulate local immune cells of intestinal tracts to generate secretory IgA, stimulate an organism to generate mucosal reaction, and further stimulate the organism to generate cellular immunity and humoral immunity through blood circulation.
Owner:QINGDAO AGRI UNIV
Vaccine composition against swine fever virus and porcine circovirus infection and its preparation and application
ActiveCN103623403BHigh titerSimple production processViral antigen ingredientsAntiviralsVaccine ProductionSwine Fever Virus
Owner:PU LIKE BIO ENG
A kind of vaccine composition and its preparation method and application
ActiveCN104511015BImprove immunitySatisfactory immune effectAntibacterial agentsAntiviralsAntigenImmune effects
The invention provides a vaccine composition, which comprises: immunizing amount of classical swine fever virus antigen, immunizing amount of porcine pseudorabies virus antigen and immunizing amount of Haemophilus parasuis antigen. The vaccine composition is safe to use, has controllable quality, good immune effect, and the protection rate of the virus challenge reaches 80-100%. Satisfactory immune effect can be achieved by intranasally immunizing 0-3 day-old piglets and intramuscularly immunizing 50-day-old piglets.
Owner:PULIKE BIOLOGICAL ENG INC
Preparation method of hog cholera virus antigen for improving virus content or reaction rate of animal body
PendingCN112843226AIncrease response rateImprove heat response rate of stylingSsRNA viruses positive-senseViral antigen ingredientsClassical swine fever virus CSFVAntigen
The invention discloses a preparation method of a hog cholera virus antigen for improving the virus content or reaction rate of an animal body. The method comprises the steps: 1) grinding, diluting and filtering a hog cholera virus production seed virus for later use; (2) inoculating the filtrate (namely the hog cholera lapinized virus strain virus suspension) into rabbit bodies in at least two inoculation modes; (3) after inoculating for 24 hours, measuring the temperature once every 6 hours, and recording the temperature of each rabbit body; (4) collecting splenic tissues of shaped hot rabbit bodies after 24-48 hours; 5) immunizing the rest rabbit bodies which is not shaped and hot within 48 hours with the filtrate (namely the hog cholera lapinized virus strain virus suspension) again, wherein the inoculation mode is the same as that of the step 2); 6) measuring the temperature once every 6 hours after the second inoculation, and recording the temperature of each rabbit body; and 7), collecting splenic tissues of rabbit bodies which is shaped and hot 24-48 hours after secondary inoculation is conducted, and thus obtaining the hog cholera virus antigen. According to the method, the method of being more than 1 immunization and more than one immunization way is adopted, the viruses are propagated, virus-containing tissues are collected, the animal body utilization rate is increased to 95% or above, and the virus content is increased by 1.5-6 times or above compared with the prior art.
Owner:兆丰华生物科技(南京)有限公司 +3
Chog cholera and porcine pseudorabies bivalent vaccine as well as preparation method and application thereof
PendingCN114272368AAvoid immune interferenceHigh speedViral antigen ingredientsMicroorganism based processesClassical swine fever virus CSFVRabies
The invention discloses a swine fever and swine pseudorabies bivalent vaccine, which is prepared by inserting a swine pseudorabies virus gB antigen and a swine fever virus E2 antigen into a replication-defective chimpanzee adenovirus shuttle vector, the gene sequence of the swine pseudorabies virus gB antigen is SEQ ID No.1, and the gene sequence of the swine fever virus E2 antigen is as shown in SEQ ID No.2. The preparation method comprises the following steps: 1) preparing a classical swine fever virus antigen protein E2 gene and a porcine pseudorabies virus antigen protein gB gene; (2) constructing a recombinant replication-deficient chimpanzee adenovirus shuttle vector; (3) obtaining replication-defective chimpanzee adenovirus plasmids; and (4) obtaining the recombinant adenovirus with a uniform genome structure, and preparing the recombinant adenovirus into the vaccine with the required dosage form as required. According to the invention, the immunosuppression phenomenon occurring when the porcine pseudorabies virus and the hog cholera virus are simultaneously used as antigens for immunization can be avoided, and an unexpected effect that an immune receptor can generate a hog cholera antibody within 7 days of immunization is obtained.
Owner:成都博宠生物科技有限公司
African swine fever virus chimeric protein, vaccine composition, preparation method and application thereof
ActiveCN113388040BSolve the problem of poor immunogenicityGood immune effectViral antigen ingredientsAntibody mimetics/scaffoldsClassical swine fever virus CSFVEpitope
The present invention provides an African swine fever virus chimeric protein, the chimeric protein comprises: (1) African swine fever virus p72 domain I; (2) African swine fever virus p72 domain II; (3) African swine fever virus viral p72 domain III; and (4) African swine fever virus antigenic protein. The chimeric protein provided by the present invention uses the African swine fever virus p72 protein as the backbone for the first time, and better displays the antigenic epitopes of the African swine fever virus antigenic proteins p54, p30, CD2v and p12, has good immune effect, and can produce significant Humoral and cellular immunity.
Owner:PU LIKE BIO ENG
A kind of vaccine composition and its preparation method and application
ActiveCN103041384BEliminate interferenceEliminate immune interferenceViral antigen ingredientsAntiviralsAntigenHighly pathogenic
The invention provides a vaccine composition. The vaccine composition comprises hog cholera virus antigen and porcine reproductive and respiratory syndrome (PRRS) antigen, wherein the hog cholera virus antigen is a hog cholera rabbit attenuated strain, the PRRS antigen is a high-pathogenicity PRRS virus, and the content ratio of the PRRS antigen and the hog cholera virus antigen is 100:1-100:10. The vaccine composition can eliminate the interference between the two types of antigen, achieves the purpose of preventing two infectious diseases by one injection, and greatly reduces immunization times and animal stress response.
Owner:PULIKE BIOLOGICAL ENG INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com