Vaccine composition against swine fever virus and porcine circovirus infection and its preparation and application
A porcine circovirus and swine fever virus technology, applied in antiviral agents, virus antigen components, medical preparations containing active ingredients, etc., can solve the problems of cumbersome seed virus preparation process, reduced immune effect, and large batch-to-batch differences , to avoid immune stress response and reduce the immune effect, simplify the production process, and achieve the effect of high titer content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] 1) preparation of classical swine fever virus antigen;
[0037] 2) Preparation of porcine circovirus type 2 subunit antigen;
[0038] 3) Antigens of classical swine fever virus and subunit antigens of porcine circovirus type 2 are mixed in proportion and then freeze-dried to prepare a dual vaccine composition against infection of classical swine fever virus and porcine circovirus type 2;
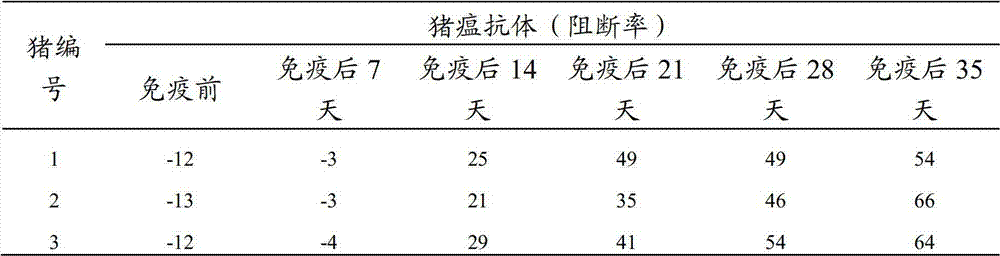
[0039] 4) Efficacy evaluation was carried out after immunizing animals with the dual vaccine composition against classical swine fever virus and porcine circovirus type 2 infection and the two single vaccines respectively.
[0040] Scheme 2 is to use the prepared porcine circovirus type 2 whole virus antigen inactivated vaccine as a diluent to dilute the swine fever virus vaccine to obtain a dual vaccine composition resistant to swine fever virus and porcine circovirus type 2 infection. Prepare the double combination vaccine composition test kit of anti-swine fever virus and porcine ...
Embodiment 1
[0050] Example 1: Lyophilize after mixing CSFV and porcine circovirus type 2 subunit antigens to prepare a dual vaccine composition against CSFV and porcine circovirus type 2 infection
[0051] 1. Preparation of CSFV antigen solution
[0052] (1) The highly sensitive ST cells (purchased from ATCC) (American type culture collection, American type culture collection) grown into a good monolayer were treated with 0.125wt% trypsin and 0.03wt% EDTA ( ethylenediaminetetraacetic acid) digestion liquid was digested and dispersed, counted cells, and inoculated with a suitable density, added MEM containing 3wt% to 5wt% FBS (fetal bovine serum) (purchased from PAA company, batch number A15110-1462) (purchased from GIBCO Company, batch number 856833), and at the same time add seed virus according to M.O.I. (multiplicity of infection) = 0.1-0.2 exposure dose, and place in a 34-37°C incubator for cultivation.
[0053] (2) After culturing for three days, the first poisoning was carried out,...
Embodiment 2
[0089] Embodiment 2: take the prepared porcine circovirus type 2 whole virus antigen inactivated vaccine as the diluent, dilute the mode of the dual vaccine composition of swine fever virus vaccine and obtain anti-swine fever virus and porcine circovirus type 2 infection To prepare a dual vaccine composition kit against classical swine fever virus and porcine circovirus type 2 infection.
[0090] 1. Preparation of CSFV antigen solution
[0091] (1) Digest and disperse ST cells (derived from ATCC) that have grown into a good monolayer and are highly sensitive to classical swine fever virus with a digestive solution of 0.125% trypsin and 0.03% EDTA, count the cells and inoculate them at an appropriate density , add 3wt% ~ 5wt% FBS (purchased from PAA company, batch number A15110-1462) MEM (purchased from GIBCO, batch number 856833), at the same time add seed poison according to M.O.I. cultured in an incubator.
[0092] (2) After culturing for three days, the first poisoning wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com