B cell epitope peptide segment of amino-terminal pro-brain natriuretic peptide and applications thereof
An amino-terminal, brain natriuretic peptide technology, applied in the medical field, can solve the problems of difficult patient treatment, insufficient specificity and timeliness, and inability to assess the long-term impact of myocardial damage, and achieve high titer, good specificity and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
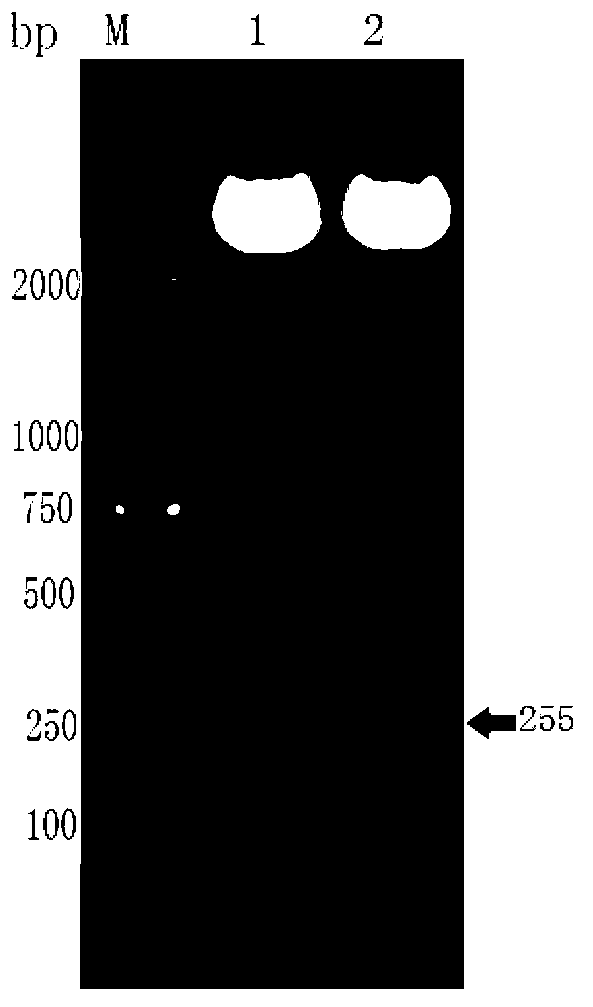
[0028] Embodiment 1 recombinant NT-proBNP gene cloning
[0029] The human NT-proBNP coding gene sequence was obtained from GenBank (accession number NCBI Reference Sequence: NM_002521.2), and submitted to the biological online analysis software GCUA (Graphical codon usage analyzer, http: / / guca.schoedl.del) for analysis and evaluation, And carry out codon bias tropism transformation and optimization. The optimization method is to synonymously replace codons whose expression activity of NT-proBNP protein is less than 20% in E. coli with codons preferred by E. coli (the same function can also be achieved without codon replacement). SEQ ID No: 1 is the nucleotide sequence of the gene encoding human NT-proBNP obtained from GenBank, and the underlined part is the codon whose expression activity is lower than 20% in Escherichia coli according to the results of GCUA analysis.
[0030] cacccgctgggcagc ccc ggt tca gcc tcg gac ttg gaaacg tccgggtta caggagcagcgcaaccat ttg cagg...
Embodiment 2
[0041] Example 2 Recombinant pET42a-NT-proBNP plasmid construction
[0042] The pET42a vector and the whole gene synthesis sequence subcloning vector (see Example 1 for details) were digested by Sal I and BamH I for 4 hours, recovered by agarose gel electrophoresis, and washed with T 4 DNA ligase was ligated overnight at 4°C. Put 200 μL of the prepared competent DH5α bacteria into the ice bath, pipette 1 μL of the ligation product into the tube, transform the DH5α bacteria, pat and mix, ice bath for 30 minutes, 42°C water bath for 90 seconds, take out the centrifuge tube and ice bath for 2 minutes, Add 800 μL of 2×YT culture medium at room temperature and mix well, shake and culture at 37°C at 220 rpm for 1 hour, and apply 50 μL, 200 μL and all remaining transformed bacteria to three 2×YT cultures containing kanamycin resistance Cultivate overnight on the plate in a constant temperature incubator at 37°C. The next day, the albino-free colonies were picked and inoculated in LB...
Embodiment 3
[0043] Example 3 Induced expression of recombinant NT-proBNP protein
[0044] Transform BL21 codon plus (DE3) engineering bacteria by conventional methods, take the recombinant pET42a-NT-proBNP plasmid to transform BL21 codon plus (DE3) bacteria, spread on LB solid medium containing chloramphenicol resistance and kanamycin resistance , cultivated overnight in a 37°C incubator, picked white colonies and inoculated them in LB medium for expanded culture the next day, cultured at 30°C, and added IPTG with a final concentration of 0.6mmol / L to induce expression for 4 hours when the measured bacterial OD value reached 0.6-0.8 . After cultivation, each gram of wet bacteria was resuspended with 10 times the volume of PBS buffer (pH 7.3), mixed evenly, and ultrasonically destructed. After the bacterium was completely broken, it was centrifuged at 10,000 rpm at 4°C for 15 minutes, and the supernatant was filtered with a 0.45 μm microporous membrane. . Take the supernatant and precipi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com