B cell epitope peptide of human neutrophil gelatinase associated lipocalin and application thereof
A lipocalin and neutrophil technology, applied in the medical field, can solve problems such as affecting the application of AKI diagnosis and failing to develop antibodies, and achieve the effects of high titer, good specificity and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
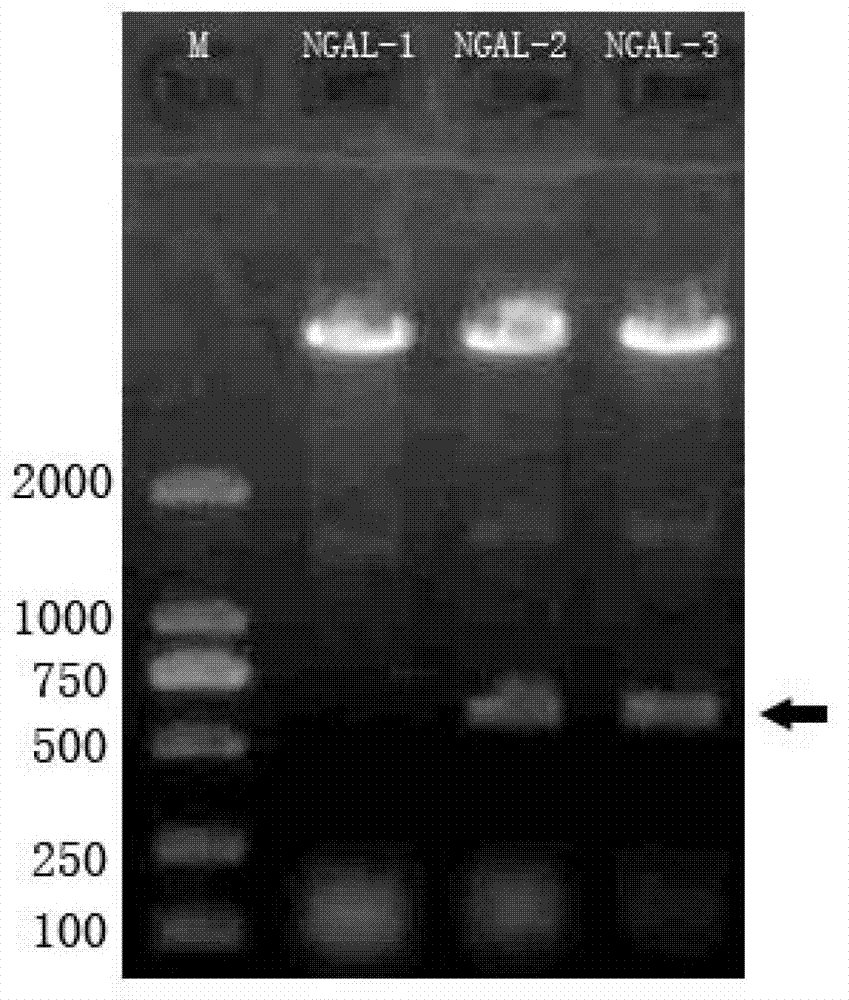
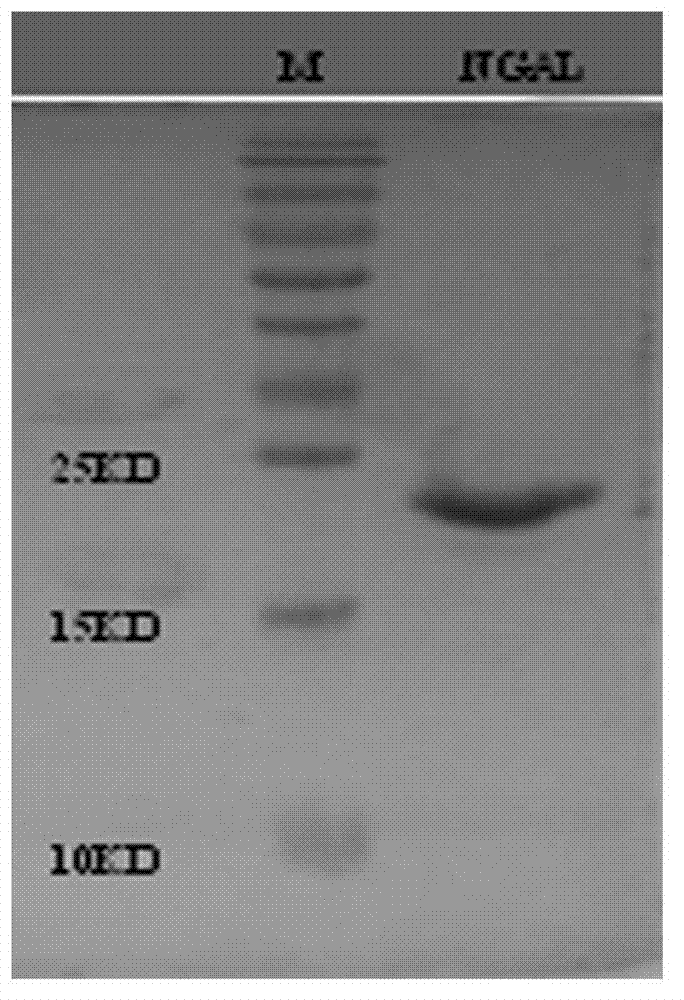
[0032] The preparation of embodiment 1NGAL recombinant protein
[0033] On the basis of obtaining the NGAL coding gene from GENBANK, carry out codon preference modification, chemically synthesize the coding gene fragment, clone it into a prokaryotic expression vector and induce protein expression after DNA sequencing and identification, and then purify and prepare NGAL recombinant protein in large quantities. The protein can be used as an immunogen and screening agent for antibody preparation, and can also be used as a calibrator for the establishment of NGAL quantitative detection in subsequent experiments, specifically:
[0034] One NGAL recombinant protein gene clone:
[0035] Obtain the gene sequence of human NGAL protein from GENBANK (accession number is NM 005564.3), and submit it to Graphical codon usage analyzer (http: / / guca.schoedl.del) to analyze its codon bias; specifically, human The synonymous replacement of NGAL gene codons with a usage rate of <10% in E. coli i...
Embodiment 2
[0058] Example 2 NGAL molecular B cell epitope prediction and polypeptide synthesis
[0059] The sequence of the chemically synthesized B cell epitope peptide is obtained from the results of bioinformatics analysis, and the NGAL protein sequence is obtained from the NCBI Protein database, and the sequence is shown in SEQ ID NO:2. Comprehensively analyze the secondary structure, antigenicity, hydrophilicity and hydrophobicity, accessibility, flexibility and possible glycosylation sites of NGAL protein, analyze and score each segment, and select the region with a high score as the B cell epitope region. Specifically, use Chou & Fasman to predict β-turn angle, Emini method to predict antigen surface accessibility, Karplus & Schulz method to predict protein flexibility, Kolaskar & Tongaonkar protein antigenicity analysis, Parker method protein hydrophobicity analysis, Bepipred linear epitope prediction and The glycosylation information of NGAL was obtained from the UniProt databas...
Embodiment 3
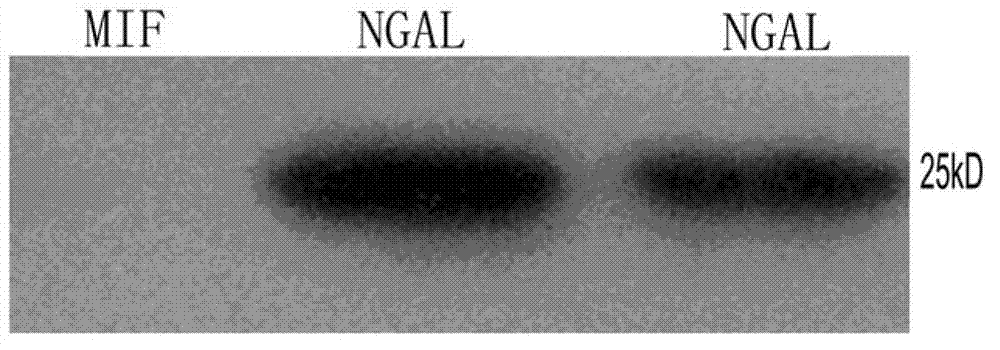
[0062] Example 3 Preparation and Identification of Monoclonal Antibody
[0063] Immunization of Balb / c mice with a peptide derivative of NGAL B cell epitope
[0064] The NGAL B cell epitope peptide antigen obtained in Example 2 was taken out from the -80°C refrigerator as an antigen, dissolved and then filtered. Female Balb / c mice aged 6 weeks and weighing about 20 g were selected for immunization. Antigen emulsification adopts double syringe mutual pushing method. For the first immunization, the NGAL B cell epitope peptide antigen shown in SEQ ID NO: 3 was emulsified and mixed with an equal volume of Freund's complete adjuvant to obtain the antigen mixture, and each mouse was intradermally multi-pointed in an amount of 100 μg Plus intraperitoneal injection. The second and third immunizations were carried out on the 14th and 28th days respectively. The adjuvant was changed to incomplete Freund's adjuvant. The amount of antigen, injection volume and route remained unchanged....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com