Helicobacter Pylori urease B subunit Th epitope peptide, its coding DNA, vaccine and uses
A technology of Helicobacter pylori and urease, which is applied in the field of medicine and biology, can solve the problems of economic difficulties of drug therapy, large toxic and side effects of drug therapy, treatment failure, etc., and achieves good application prospects, good immunogenicity, and good specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1, screening identification of Th epitope:
[0099] 1.1 Prediction and synthesis of epitope peptides
[0100] First, the UreB sequence of Hp was searched in the NCBI protein database. UreB is conserved in various strains. The UreB amino acid sequence of one of the strains, Helicobacter pylori 26695 (No. NP 206872) was selected for prediction. The UreB sequence has a total of 569 amino acids. The online software RANKPEP software was used to analyze the amino acid sequence of UreB of Hp, and seven possible Th epitope peptides were screened, and seven peptides were prepared by conventional peptide total artificial synthesis (assisted by Beijing Zhongke Yaguang Co., Ltd.). The purity of the polypeptides that may induce the body to produce Th response is above 85%. See Table 1 for information on the synthesized peptides.
[0101] NCBI's protein database search URL:
[0102] http: / / www.ncbi.nlm.nih.gov / entrez / querv.fcgi? db=Protein&itool=toolbar
[0103] RAN...
Embodiment 2
[0111] Example 2: Detection of epitope peptide-induced polarization direction of Th cells by ELISA method:
[0112] Spleen cells from mice immunized with rUreB were collected, and spleen CD4 + For T lymphocytes, resuspend the cells with RPMI-1640 complete medium, place in a 24-well flat-bottomed culture plate, and add CD4 to each well + T lymphocytes 5×10 5 ,Co 60Irradiated (20Gy) splenic mononuclear cells (as antigen-presenting cells) 5×10 5 , while adding the diluted synthetic peptide (final concentration 1.25 μg / ml), no synthetic peptide was added to negative control wells, the final volume was 0.5 ml / well, and 3 parallel wells were made for each group. 72 hours after the mouse spleen lymphocytes were stimulated by the synthetic epitope peptide, 400ul of the culture supernatant was collected and stored at -70°C for later use. The Th1 / Th2 typing kit from eBIOSCIENCE was used to detect cytokines. The specific steps are as follows: Coat the microtiter plate with gamma int...
Embodiment 3
[0114] Example 3: Co-stimulatory effect of epitope peptides
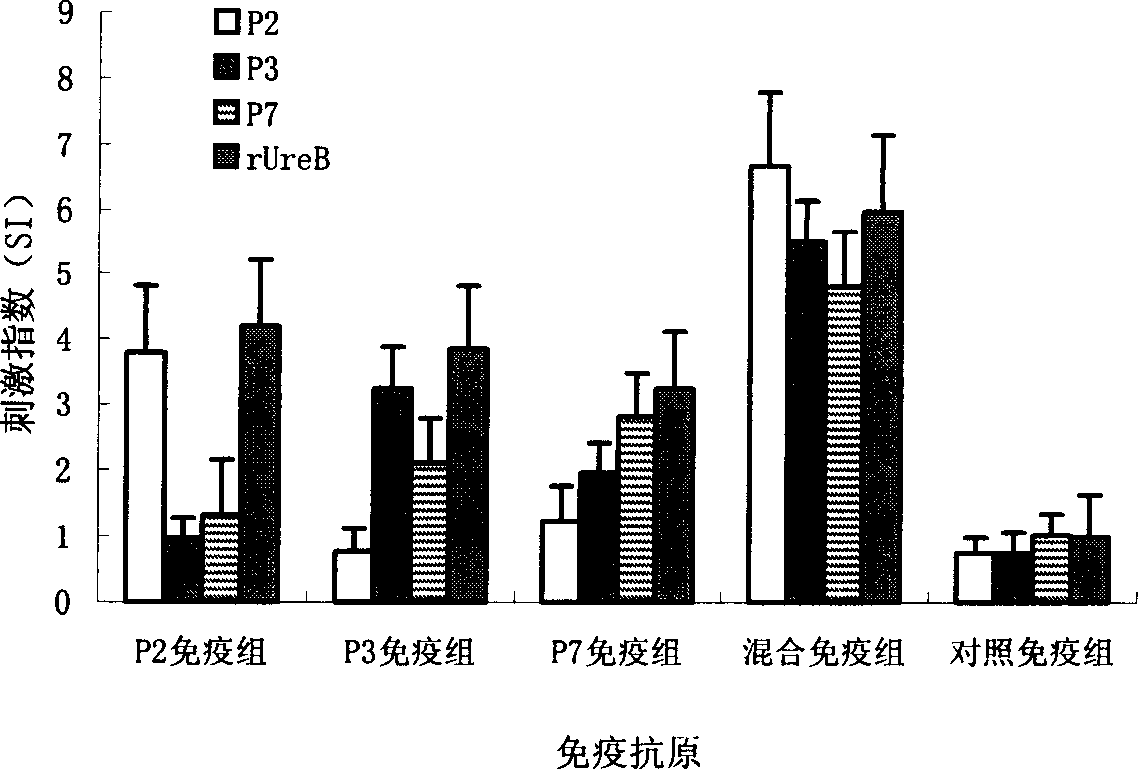
[0115] The specific operation is the same as in Example 1, the difference is that in addition to using a separate synthetic epitope peptide to stimulate lymphocytes, the three selected epitope peptides are also mixed in pairs or all as stimulators to stimulate lymphocyte proliferation. The final total concentration of the peptides was 1.25 μg / ml, and the rest of the steps were the same to compare whether there was any difference between the stimulation effect of the combination of epitope peptides and the stimulation effect of individual epitope peptides.
[0116] Results: The stimulating effects of the combination of three epitope peptides in pairs and the three mixtures were higher than those of individual epitope peptide stimulation (see Table 2), suggesting that the three epitopes can be combined in the subsequent vaccine design.
[0117] epitope peptide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com