Compositions and methods for the prevention and treatment of cancer
A cancer, complex technology, applied in the therapeutic field of cancer prevention and treatment, can solve the problems of expansion of circulating cytotoxic T cell lymphocytes, inability to produce costimulatory molecules, failure of cancer immunotherapy strategies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0142] Nanoparticles can be formed by contacting an aqueous phase containing antigen / MHC / co-stimulatory molecule complexes and polymers with a non-aqueous phase, followed by evaporation of the non-aqueous phase to allow aggregation of the particles in the aqueous phase, As taught in US Patent No. 4,589,330 or 4,818,542. Preferred polymers for this preparation method are natural or synthetic copolymers or polymers selected from the group consisting of gelatin agar, starch, arabinogalactan, albumin, collagen, polyglycolic acid, polylactic acid, glycolide -L(-)-lactide poly(ε-caprolactone, poly(ε-caprolactone-CO-lactic acid) copolymer, poly(ε-caprolactone-CO-glycolic acid) copolymer, poly( β-hydroxybutyrate), poly(ethylene oxide), polyethylene, poly(alkyl-2-cyanoacrylate), poly(hydroxyethyl methacrylate), polyamide, polyamino acid, poly(2 -hydroxyethyl DL-asparagine), polyester urea, poly(L-phenylalanine / ethylene glycol / 1,6-hexamethylene diisocyanate) and poly(methyl methacrylat...
Embodiment 1
[0171]Synthesis and characterization of gold-based pMHC-NPs. Gold nanoparticles (GNPs) of specific sizes can be synthesized according to Levy, R. et al. ("Rational and combinatorial design of peptide capping ligands for gold nanoparticles." J Am Chem Soc 126, 10076-84 (2004)). The size, density, charge and monodispersity of the GNP preparations were measured using a spectrophotometer, transmission electron microscopy (TEM) and dynamic light scattering. Then, GNP samples were concentrated and conjugated to pHMC (antigen-MHC complexes) using different methods. Developed for quantification of pMHC valence / GNP and for high concentration (~10 14 / ml) method of concentrating antigen=MHC-GNP without disrupting monodispersity ( figure 1 ).
Embodiment 2
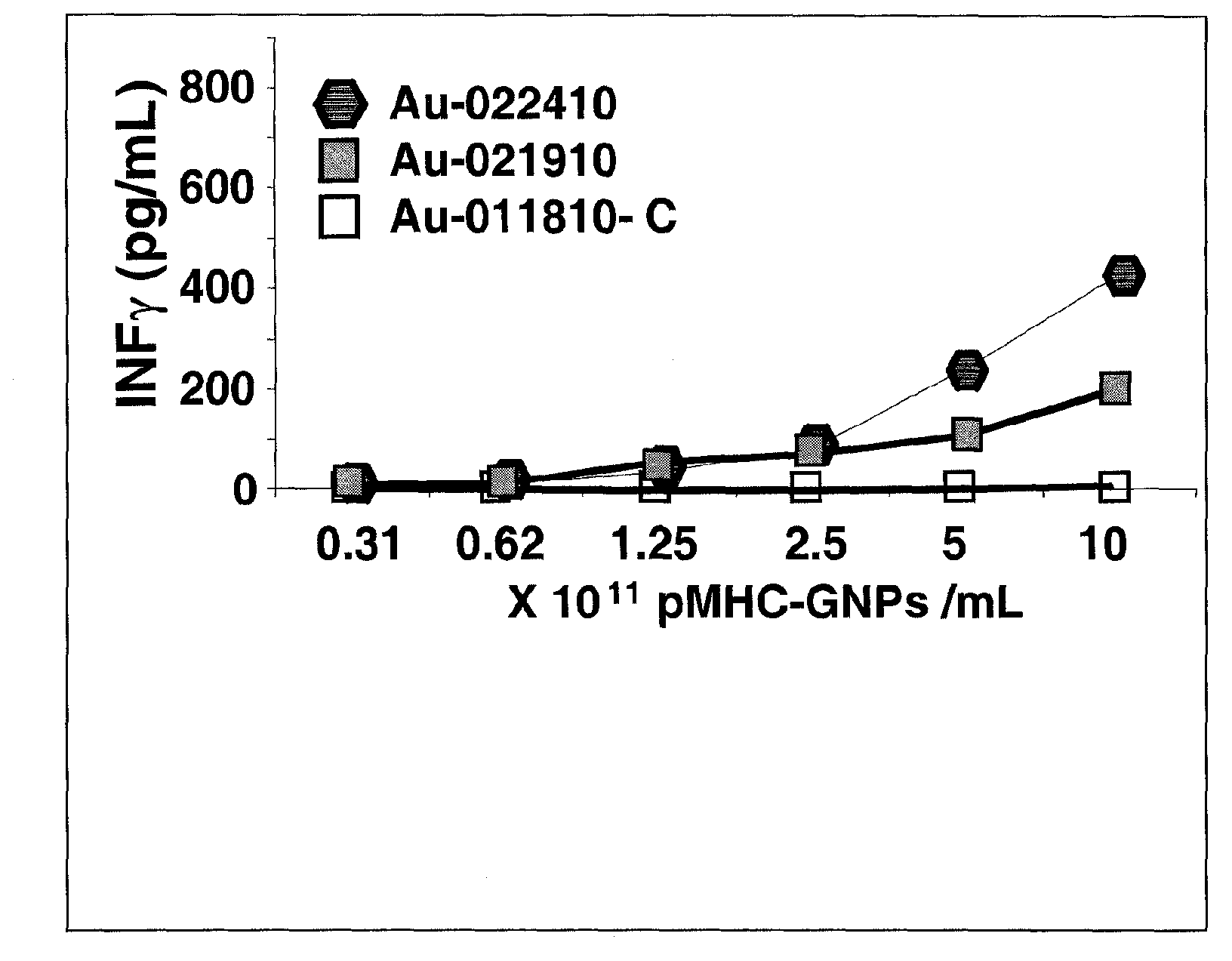
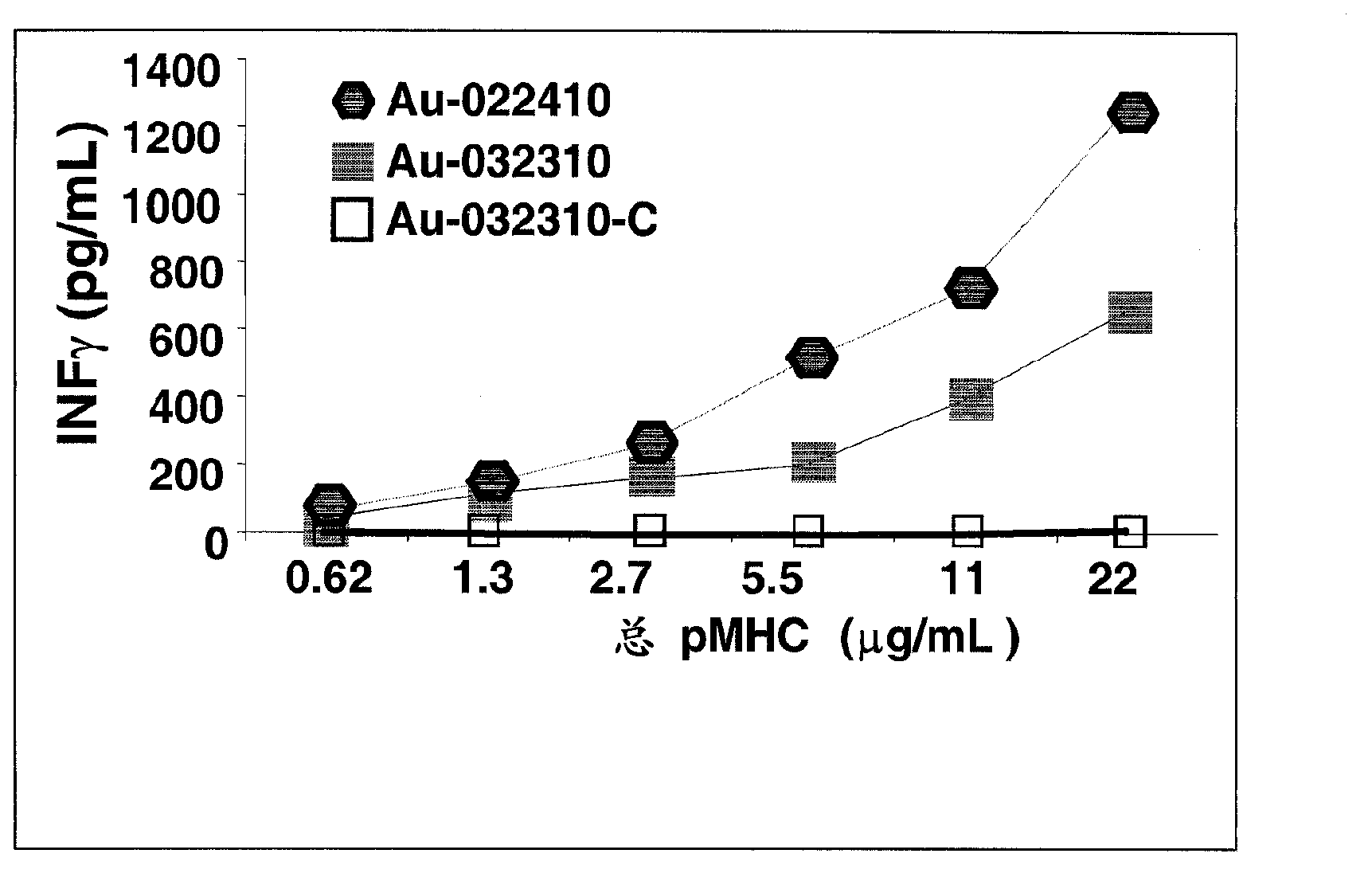
[0173] pMHC binding capacity of GNP. Two methods were used to coat pMHC onto GNPs of different sizes: (i) random binding of pMHC to GNPs through electrostatic interactions; and (ii) sulfhydryl-PEG-NH 2 Linker-directed binding. In this case, additional thiol-PEG was used as a GNP stabilizer to prevent aggregation. It is contemplated that the first approach would result in very high ligand densities, destroying the specificity of pMHC binding (ie, only a fraction of pMHC can be recognized by cognate T cells). The second approach aims to generate pMHC-GNPs that carry less pMHC but bind directionally through their C-terminus. Both methods were tested against 14nm GNP and 40nm GNP. The pHMC binding capacity of GNPs was confirmed to be a function of surface area for both methods. Therefore, when the nanoparticle size is larger, more pMHC is bound. Unexpectedly, it was found that PEG-mediated binding not only ensures the directionality of binding but also enhances the binding ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com