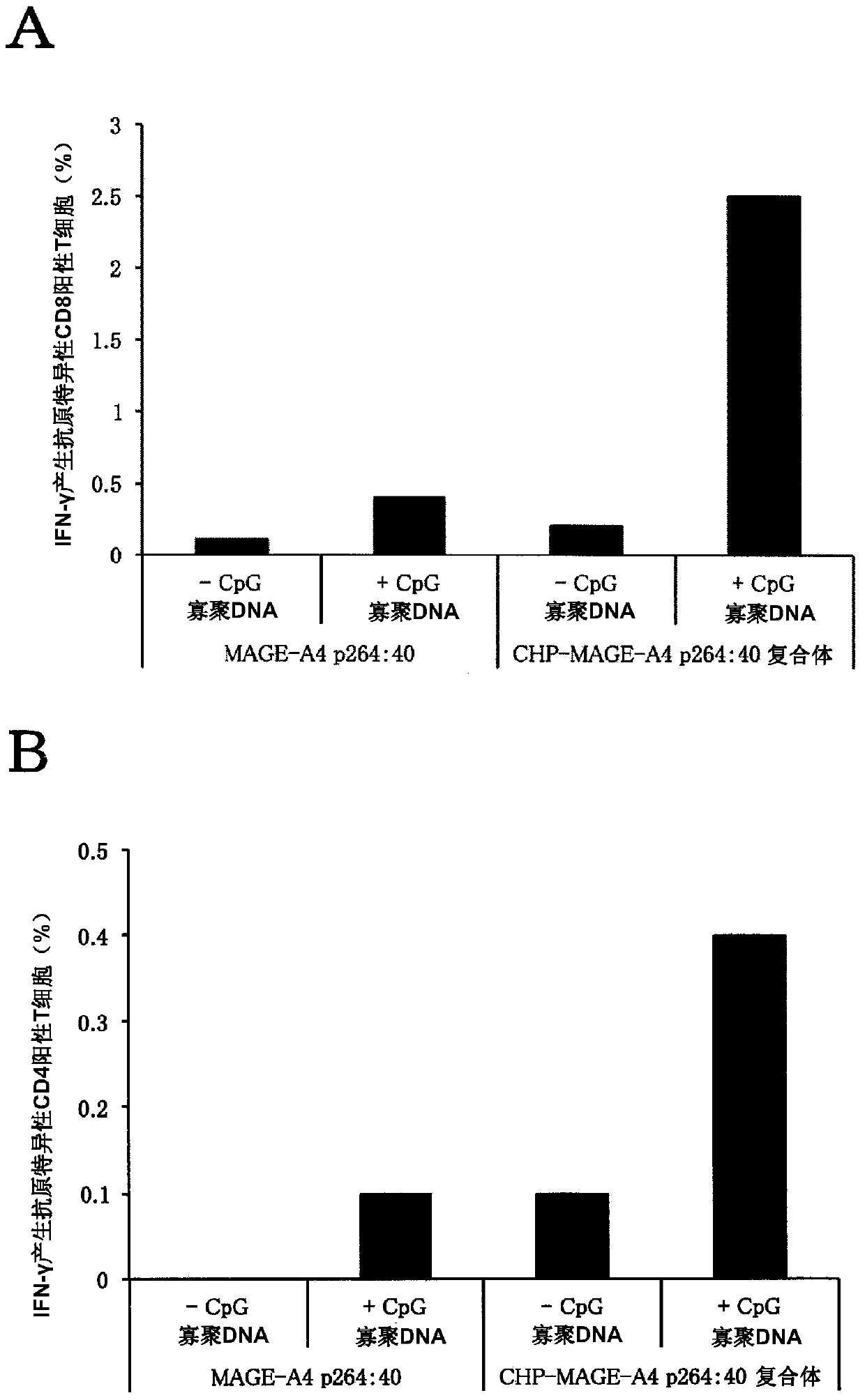
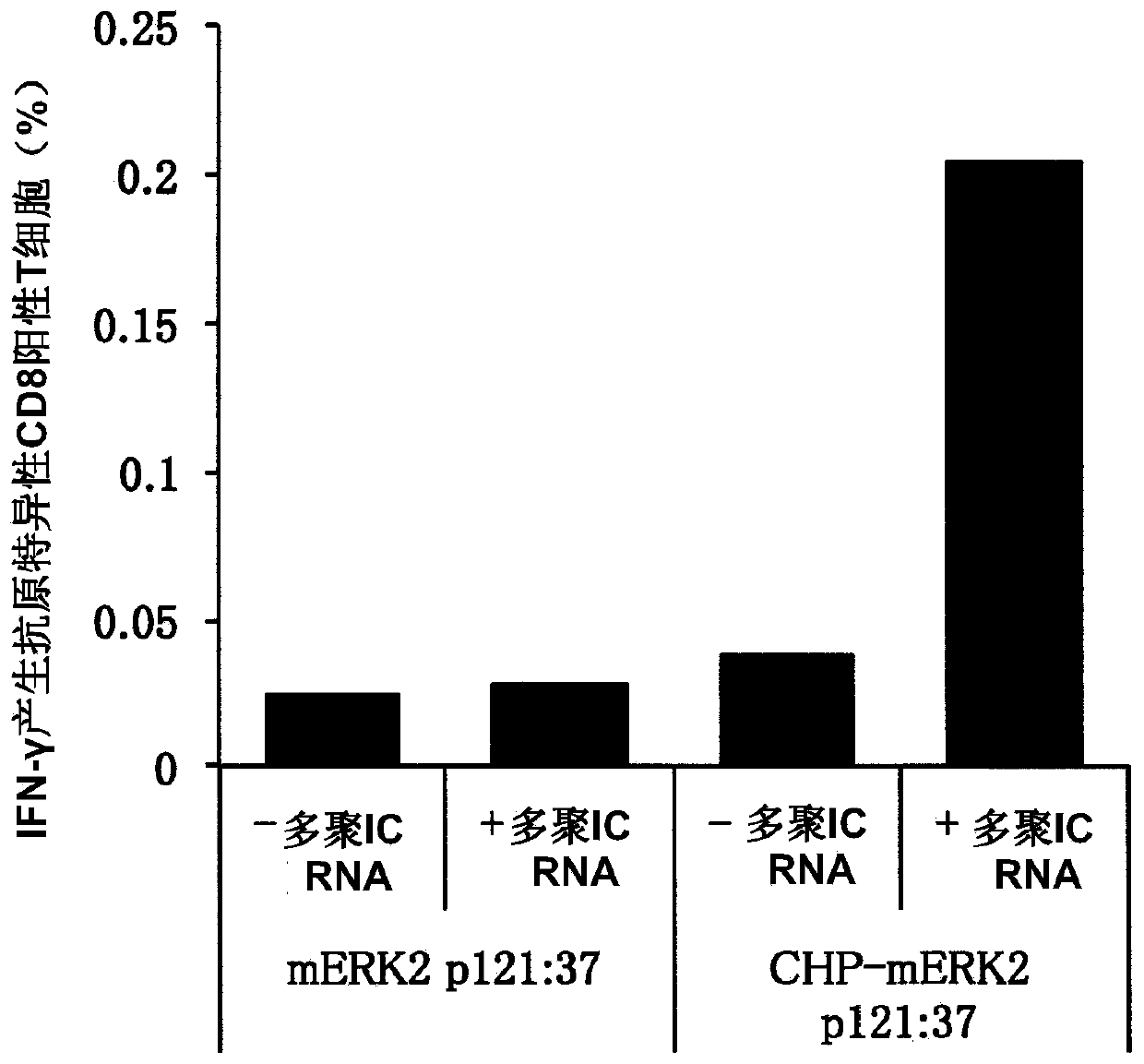
Vaccine preparation for cancer treatment
A cancer treatment and vaccine technology, which can be applied to vaccines, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve many problems such as unclear conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0078] Next, embodiments of the present invention will be described with reference to the drawings, but the technical scope of the present invention is not limited by these embodiments, and can be implemented in various forms without changing the gist of the invention. In addition, the technical scope of the present invention extends to an equal range.
[0079]
[0080] (1) Material
[0081] BALB / c mice (female, 5 weeks old) were purchased from SLC, Japan, and bred at the Animal Center, Faculty of Medicine, Mie University. It was used for experiments after acclimatization for 1 week. The experimental plan regarding the use of mice was approved by the Ethics Committee of Mie University Faculty of Medicine. Synthetic long peptides were purchased from GenScript, Scrum or Biologica. The sequence of the synthetic long peptide is as follows: MAGE-A4p264:40 (amino acid sequence (SEQ ID: 1)): GSNPARYEFLWGPRALAETSYVKVLEHVVRVNARVRIAYP, including 9 amino acids from S at position 2 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com